Nonchimeric HLA-Identical Renal Transplant Tolerance: Regulatory Immunophenotypic/Genomic Biomarkers
- PMID: 26227106
- PMCID: PMC4718825
- DOI: 10.1111/ajt.13416
Nonchimeric HLA-Identical Renal Transplant Tolerance: Regulatory Immunophenotypic/Genomic Biomarkers
Abstract
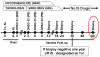
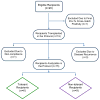
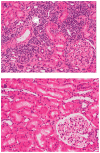
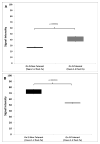
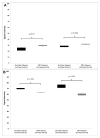
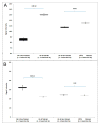
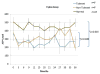
We previously described early results of a nonchimeric operational tolerance protocol in human leukocyte antigen (HLA)-identical living donor renal transplants and now update these results. Recipients given alemtuzumab, tacrolimus/MPA with early sirolimus conversion were multiply infused with donor hematopoietic CD34(+) stem cells. Immunosuppression was withdrawn by 24 months. Twelve months later, operational tolerance was confirmed by rejection-free transplant biopsies. Five of the first eight enrollees were initially tolerant 1 year off immunosuppression. Biopsies of three others after total withdrawal showed Banff 1A acute cellular rejection without renal dysfunction. With longer follow-up including 5-year posttransplant biopsies, four of the five tolerant recipients remain without rejection while one developed Banff 1A without renal dysfunction. We now add seven new subjects (two operationally tolerant), and demonstrate time-dependent increases of circulating CD4(+) CD25(+++) CD127(-) FOXP3(+) Tregs versus losses of Tregs in nontolerant subjects (p < 0.001). Gene expression signatures, developed using global RNA expression profiling of sequential whole blood and protocol biopsy samples, were highly associative with operational tolerance as early as 1 year posttransplant. The blood signature was validated by an external Immune Tolerance Network data set. Our approach to nonchimeric operational HLA-identical tolerance reveals association with Treg immunophenotypes and serial gene expression profiles.
Trial registration: ClinicalTrials.gov NCT00619528.
Keywords: Biomarker; bone marrow/hematopoietic stem cell transplantation; clinical research/practice; clinical trial; genomics; immunobiology; kidney transplantation/nephrology; monitoring: immune; tolerance: clinical; translational research/science.
© Copyright 2015 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures





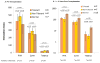
Similar articles
-
HLA identical non-chimeric and HLA disparate chimeric renal transplant tolerance.Clin Transpl. 2013:145-56. Clin Transpl. 2013. PMID: 25095503
-
Updated follow-up of a tolerance protocol in HLA-identical renal transplant pairs given donor hematopoietic stem cells.Hum Immunol. 2018 May;79(5):277-282. doi: 10.1016/j.humimm.2018.01.010. Epub 2018 Feb 1. Hum Immunol. 2018. PMID: 29408689 Free PMC article. Review.
-
Genomic biomarkers correlate with HLA-identical renal transplant tolerance.J Am Soc Nephrol. 2013 Sep;24(9):1376-85. doi: 10.1681/ASN.2013010068. Epub 2013 Jun 20. J Am Soc Nephrol. 2013. PMID: 23787913 Free PMC article.
-
Clinical strategy for induction of transplantation tolerance through mixed chimerism.Clin Transpl. 2013:127-34. Clin Transpl. 2013. PMID: 25095501
-
Immune monitoring of transplant patients in transient mixed chimerism tolerance trials.Hum Immunol. 2018 May;79(5):334-342. doi: 10.1016/j.humimm.2017.12.011. Epub 2017 Dec 28. Hum Immunol. 2018. PMID: 29289741 Free PMC article. Review.
Cited by
-
Foxp3+ regulatory T cell therapy for tolerance in autoimmunity and solid organ transplantation.Front Immunol. 2022 Nov 17;13:1055466. doi: 10.3389/fimmu.2022.1055466. eCollection 2022. Front Immunol. 2022. PMID: 36466912 Free PMC article. Review.
-
Early expansion of donor-specific Tregs in tolerant kidney transplant recipients.JCI Insight. 2018 Nov 15;3(22):e124086. doi: 10.1172/jci.insight.124086. JCI Insight. 2018. PMID: 30429370 Free PMC article.
-
Cell therapeutic approaches to immunosuppression after clinical kidney transplantation.Pediatr Nephrol. 2018 Feb;33(2):199-213. doi: 10.1007/s00467-017-3599-2. Epub 2017 Feb 23. Pediatr Nephrol. 2018. PMID: 28229281 Review.
-
Tolerance induction with donor hematopoietic stem cell infusion in kidney transplantation: a single-center experience in China with a 10-year follow-up.Ann Transl Med. 2020 Nov;8(21):1378. doi: 10.21037/atm-20-2502a. Ann Transl Med. 2020. PMID: 33313123 Free PMC article.
-
Transient increase of activated regulatory T cells early after kidney transplantation.Sci Rep. 2019 Jan 31;9(1):1021. doi: 10.1038/s41598-018-37218-x. Sci Rep. 2019. PMID: 30705299 Free PMC article.
References
-
- Gerrits JH, van de Wetering J, Weimar W, van Besouw NM. T-cell reactivity during tapering of immunosuppression to low-dose monotherapy prednisolone in HLA-identical living-related renal transplant recipients. Transplantation. 2009;87(6):907–14. - PubMed
-
- van de Wetering J, Gerrits JH, van Besouw NM, Ijzermans JNM, Weimar W. Successful tapering of immunosuppression to low-dose monotherapy steroids after living-related human leukocyte antigen-identical renal transplantation. Transplantation. 2009;87(5):740–4. - PubMed
-
- Spitzer TR, Delmonico F, Tolkoff-Rubin N, McAfee S, Sackstein R, Saidman S, et al. Combined histocompatibility leukocyte antigen-matched donor bone marrow and renal transplantation for multiple myeloma with end stage renal disease: the induction of allograft tolerance through mixed lymphohematopoietic chimerism. Transplantation. 1999;68(4):480–4. - PubMed
-
- Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Millan MT, Shizuru JA, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358(4):362–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

