The relationship between nociceptive brain activity, spinal reflex withdrawal and behaviour in newborn infants
- PMID: 26228435
- PMCID: PMC4521152
- DOI: 10.1038/srep12519
The relationship between nociceptive brain activity, spinal reflex withdrawal and behaviour in newborn infants
Abstract
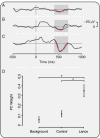
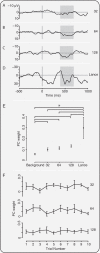
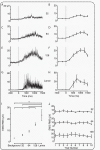
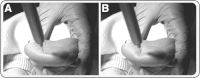
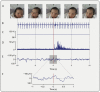
Measuring infant pain is complicated by their inability to describe the experience. While nociceptive brain activity, reflex withdrawal and facial grimacing have been characterised, the relationship between these activity patterns has not been examined. As cortical and spinally mediated activity is developmentally regulated, it cannot be assumed that they are predictive of one another in the immature nervous system. Here, using a new experimental paradigm, we characterise the nociceptive-specific brain activity, spinal reflex withdrawal and behavioural activity following graded intensity noxious stimulation and clinical heel lancing in 30 term infants. We show that nociceptive-specific brain activity and nociceptive reflex withdrawal are graded with stimulus intensity (p < 0.001), significantly correlated (r = 0.53, p = 0.001) and elicited at an intensity that does not evoke changes in clinical pain scores (p = 0.55). The strong correlation between reflex withdrawal and nociceptive brain activity suggests that movement of the limb away from a noxious stimulus is a sensitive indication of nociceptive brain activity in term infants. This could underpin the development of new clinical pain assessment measures.
Figures


References
-
- Craig K. D., Whitfield M. F., Grunau R. V., Linton J. & Hadjistavropoulos H. D. Pain in the preterm neonate: behavioural and physiological indices. Pain 52, 287–299 (1993). - PubMed
-
- Grunau R. V. & Craig K. D. Pain expression in neonates: facial action and cry. Pain 28, 395–410 (1987). - PubMed
-
- Anand K. J. & International Evidence-Based Group for Neonatal Pain. Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med. 155, 173–180 (2001). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

