Mirtazapine toxicity in cats: retrospective study of 84 cases (2006-2011)
- PMID: 26228539
- PMCID: PMC11132229
- DOI: 10.1177/1098612X15599026
Mirtazapine toxicity in cats: retrospective study of 84 cases (2006-2011)
Abstract
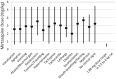
Objectives Mirtazapine is commonly used in veterinary medicine at doses of 1.88 or 3.75 mg as an appetite stimulant. The objectives of this study were to determine the most common adverse effects reported and the dose associated with these signs. Methods Records of cats with mirtazapine exposure (2006-2011) were obtained from the American Society for the Prevention of Cruelty to Animals' Animal Poison Control Center. The following parameters were recorded: signalment, weight, outcome, agent ingested, amount ingested, route of exposure, clinical signs observed, intended of use, onset time of signs and duration of signs. Results The 10 most commonly observed adverse effects reported in 84 cats exposed to mirtazapine included vocalization (56.0% of cats; mean dose 2.56 mg/kg), agitation (31.0%; 2.57 mg/kg), vomiting (26.2%; 2.92 mg/kg), abnormal gait/ataxia (16.7%; 2.87 mg/kg), restlessness (14.3%; 3.55 mg/kg), tremors/trembling (14.3%; 2.43 mg/kg), hypersalivation (13.0%; 2.89 mg/kg), tachypnea (11.9%; 3.28 mg/kg), tachycardia (10.7%; 3.04 mg/kg) and lethargy (10.7%; 2.69 mg/kg). Fifty-nine (70.2%) cases were considered accidental ingestions and 25 (29.8%) cases were given mirtazapine as prescribed. The doses associated with signs of toxicity were 15.00 mg (40 cats), 3.75 mg (25 cats), 7.50 mg (four cats), 30.00 mg (one cat), 18.75 mg (one cat), 11.25 mg (one cat), 5.80 mg (one cat) and 1.88 mg (one cat). For cats with available information, the onset of clinical signs ranged from 15 mins to 3 h, and time to resolution of clinical signs ranged from 12-48 h. Conclusions and relevance The greater number of adverse effects at 3.75 mg rather than 1.88 mg suggests that the latter may be a more appropriate starting dose for stimulating appetite while limiting toxicity. The benefit of dispensing exact doses of mirtazapine is implied given the likelihood of accidental administration of a full tablet (15 mg) and the resulting toxicity.
Conflict of interest statement
The authors do not have any potential conflicts of interest to declare.
Figures

References
-
- Freitag KA, Saker KE, Thomas E. Acute starvation and subsequent refeeding affect lymphocyte subsets and proliferation in cats. J Nutr 2000; 130: 2444–2449. - PubMed
-
- Center SA. Feline hepatic lipidosis. Vet Clin North Am Small Anim Pract 2005; 35: 225–269. - PubMed
-
- de Boer T. The pharmacologic profile of mirtazapine. J Clin Psychiatry 1996; 57 Suppl 4: 19–25. - PubMed
-
- Riechelmann RP, Burman D, Tannock IF, et al.. Phase II trial of mirtazapine for cancer-related cachexia and anorexia. Am J Hosp Palliat Care 2010; 27: 106–110. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

