Computational modeling of Adelta-fiber-mediated nociceptive detection of electrocutaneous stimulation
- PMID: 26228799
- PMCID: PMC4572082
- DOI: 10.1007/s00422-015-0656-4
Computational modeling of Adelta-fiber-mediated nociceptive detection of electrocutaneous stimulation
Abstract
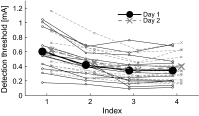
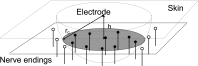
Sensitization is an example of malfunctioning of the nociceptive pathway in either the peripheral or central nervous system. Using quantitative sensory testing, one can only infer sensitization, but not determine the defective subsystem. The states of the subsystems may be characterized using computational modeling together with experimental data. Here, we develop a neurophysiologically plausible model replicating experimental observations from a psychophysical human subject study. We study the effects of single temporal stimulus parameters on detection thresholds corresponding to a 0.5 detection probability. To model peripheral activation and central processing, we adapt a stochastic drift-diffusion model and a probabilistic hazard model to our experimental setting without reaction times. We retain six lumped parameters in both models characterizing peripheral and central mechanisms. Both models have similar psychophysical functions, but the hazard model is computationally more efficient. The model-based effects of temporal stimulus parameters on detection thresholds are consistent with those from human subject data.
Keywords: Computational models; Detection threshold; Nociceptive pathway; Stimulus detection; Stimulus parameters.
Figures








References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

