Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey
- PMID: 26228813
- PMCID: PMC4854652
- DOI: 10.1038/leu.2015.212
Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey
Abstract
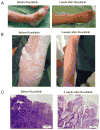
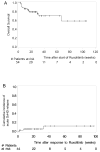
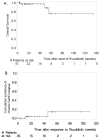
Despite major improvements in allogeneic hematopoietic cell transplantation over the past decades, corticosteroid-refractory (SR) acute (a) and chronic (c) graft-versus-host disease (GVHD) cause high mortality. Preclinical evidence indicates the potent anti-inflammatory properties of the JAK1/2 inhibitor ruxolitinib. In this retrospective survey, 19 stem cell transplant centers in Europe and the United States reported outcome data from 95 patients who had received ruxolitinib as salvage therapy for SR-GVHD. Patients were classified as having SR-aGVHD (n=54, all grades III or IV) or SR-cGVHD (n=41, all moderate or severe). The median number of previous GVHD-therapies was 3 for both SR-aGVHD (1-7) and SR-cGVHD (1-10). The overall response rate was 81.5% (44/54) in SR-aGVHD including 25 complete responses (46.3%), while for SR-cGVHD the ORR was 85.4% (35/41). Of those patients responding to ruxolitinib, the rate of GVHD-relapse was 6.8% (3/44) and 5.7% (2/35) for SR-aGVHD and SR-cGVHD, respectively. The 6-month-survival was 79% (67.3-90.7%, 95% confidence interval (CI)) and 97.4% (92.3-100%, 95% CI) for SR-aGVHD and SR-cGVHD, respectively. Cytopenia and cytomegalovirus-reactivation were observed during ruxolitinib treatment in both SR-aGVHD (30/54, 55.6% and 18/54, 33.3%) and SR-cGVHD (7/41, 17.1% and 6/41, 14.6%) patients. Ruxolitinib may constitute a promising new treatment option for SR-aGVHD and SR-cGVHD that should be validated in a prospective trial.
Conflict of interest statement
Figures


References
-
- MacMillan ML, DeFor TE, Weisdorf DJ. The best endpoint for acute GVHD treatment trials. Blood. 2010;115:5412–5417. - PubMed
-
- Wolff D, Ayuk F, Elmaagacli A, Bertz H, Lawitschka A, Schleuning M, et al. Current practice in diagnosis and treatment of acute graft-versus-host disease: results from a survey among German-Austrian-Swiss hematopoietic stem cell transplant centers. Biol Blood Marrow Transplant. 2013;19:767–776. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

