Decision Aids: The Effect of Labeling Options on Patient Choices and Decision Making
- PMID: 26229084
- PMCID: PMC4592400
- DOI: 10.1177/0272989X15598532
Decision Aids: The Effect of Labeling Options on Patient Choices and Decision Making
Abstract
Background: Conscious and unconscious biases can influence how people interpret new information and make decisions. Current standards for creating decision aids, however, do not address this issue.
Method: Using a 2×2 factorial design, we developed surveys that contained a decision scenario (involving a choice between aspirin or a statin drug to lower risk of heart attack) and a decision aid. Each aid presented identical information about reduction in heart attack risk and likelihood of a major side effect. They differed in whether the options were labeled and the amount of decisional guidance: information only (a balance sheet) versus information plus values clarification (a multicriteria decision analysis). We sent the surveys to members of 2 Internet survey panels. After using the decision aid, participants indicated their preferred medication. Those using a multicriteria decision aid also judged differences in the comparative outcome data provided for the 2 options and the relative importance of achieving benefits versus avoiding risks in making the decision.
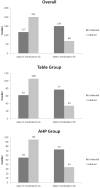
Results: The study sample size was 536. Participants using decision aids with unlabeled options were more likely to choose a statin: 56% versus 25% (P < 0.001). The type of decision aid made no difference. This effect persisted after adjustment for differences in survey company, age, gender, education level, health literacy, and numeracy. Participants using unlabeled decision aids were also more likely to interpret the data presented as favoring a statin with regard to both treatment benefits and risk of side effects (P ≤ 0.01). There were no significant differences in decision priorities (P = 0.21).
Conclusion: Identifying the options in patient decision aids can influence patient preferences and change how they interpret comparative outcome data.
Keywords: cognitive biases; patient decision aids; shared decision making.
© The Author(s) 2015.
Figures
Similar articles
-
Comparative Effectiveness of a Web-Based Patient Decision Aid for Therapeutic Options for Sickle Cell Disease: Randomized Controlled Trial.J Med Internet Res. 2019 Dec 4;21(12):e14462. doi: 10.2196/14462. J Med Internet Res. 2019. PMID: 31799940 Free PMC article. Clinical Trial.
-
Assessment of unconscious decision aids applied to complex patient-centered medical decisions.J Med Internet Res. 2015 Feb 5;17(2):e37. doi: 10.2196/jmir.3739. J Med Internet Res. 2015. PMID: 25677337 Free PMC article. Clinical Trial.
-
Balance Sheets Versus Decision Dashboards to Support Patient Treatment Choices: A Comparative Analysis.Patient. 2015 Dec;8(6):499-505. doi: 10.1007/s40271-015-0111-6. Patient. 2015. PMID: 25618789 Free PMC article. Clinical Trial.
-
Balancing the presentation of information and options in patient decision aids: an updated review.BMC Med Inform Decis Mak. 2013;13 Suppl 2(Suppl 2):S6. doi: 10.1186/1472-6947-13-S2-S6. Epub 2013 Nov 29. BMC Med Inform Decis Mak. 2013. PMID: 24625214 Free PMC article. Review.
-
Systematic Review of Decision Aids for Newly Diagnosed Patients with Prostate Cancer Making Treatment Decisions.J Urol. 2015 Nov;194(5):1247-52. doi: 10.1016/j.juro.2015.05.093. Epub 2015 Jun 6. J Urol. 2015. PMID: 26055824
Cited by
-
A global, incremental development method for a web-based prostate cancer treatment decision aid and usability testing in a Dutch clinical setting.Health Informatics J. 2019 Sep;25(3):701-714. doi: 10.1177/1460458217720393. Epub 2017 Jul 27. Health Informatics J. 2019. PMID: 28747076 Free PMC article.
-
Parent Preferences for Delaying Insulin Dependence in Children at Risk of Stage III Type 1 Diabetes.Diabetes Technol Ther. 2020 Aug;22(8):584-593. doi: 10.1089/dia.2019.0444. Epub 2020 Mar 11. Diabetes Technol Ther. 2020. PMID: 31971833 Free PMC article.
References
-
- Croskerry P. From Mindless to Mindful Practice — Cognitive Bias and Clinical Decision Making. 2013 Accessed 27 Jun 2013. - PubMed
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds D, John M, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled clinical trials. 1996;17:1–12. - PubMed
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

