Regulation of Ergothioneine Biosynthesis and Its Effect on Mycobacterium tuberculosis Growth and Infectivity
- PMID: 26229105
- PMCID: PMC4645607
- DOI: 10.1074/jbc.M115.648642
Regulation of Ergothioneine Biosynthesis and Its Effect on Mycobacterium tuberculosis Growth and Infectivity
Abstract
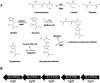
Ergothioneine (EGT) is synthesized in mycobacteria, but limited knowledge exists regarding its synthesis, physiological role, and regulation. We have identified Rv3701c from Mycobacterium tuberculosis to encode for EgtD, a required histidine methyltransferase that catalyzes first biosynthesis step in EGT biosynthesis. EgtD was found to be phosphorylated by the serine/threonine protein kinase PknD. PknD phosphorylates EgtD both in vitro and in a cell-based system on Thr(213). The phosphomimetic (T213E) but not the phosphoablative (T213A) mutant of EgtD failed to restore EGT synthesis in a ΔegtD mutant. The findings together with observed elevated levels of EGT in a pknD transposon mutant during in vitro growth suggests that EgtD phosphorylation by PknD negatively regulates EGT biosynthesis. We further showed that EGT is required in a nutrient-starved model of persistence and is needed for long term infection of murine macrophages.
Keywords: Mycobacterium tuberculosis; bacterial protein kinase; bacterial signal transduction; ergothioneine; histidine methylation; microbiology; thiol.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




Similar articles
-
Reexamination of the Ergothioneine Biosynthetic Methyltransferase EgtD from Mycobacterium tuberculosis as a Protein Kinase Substrate.Chembiochem. 2020 Oct 15;21(20):2908-2911. doi: 10.1002/cbic.202000232. Epub 2020 Jul 2. Chembiochem. 2020. PMID: 32614492
-
Inhibitors of Mycobacterium tuberculosis EgtD target both substrate binding sites to limit hercynine production.Sci Rep. 2021 Nov 15;11(1):22240. doi: 10.1038/s41598-021-01526-6. Sci Rep. 2021. PMID: 34782676 Free PMC article.
-
The evolutionary history of the genes involved in the biosynthesis of the antioxidant ergothioneine.Gene. 2014 Oct 1;549(1):161-70. doi: 10.1016/j.gene.2014.07.065. Epub 2014 Jul 26. Gene. 2014. PMID: 25068406
-
Biosynthesis of ergothioneine: current state, achievements, and perspectives.Appl Microbiol Biotechnol. 2025 Apr 12;109(1):93. doi: 10.1007/s00253-025-13476-4. Appl Microbiol Biotechnol. 2025. PMID: 40220171 Free PMC article. Review.
-
Mycobacterium tuberculosis two-component systems and implications in novel vaccines and drugs.Crit Rev Eukaryot Gene Expr. 2012;22(1):37-52. doi: 10.1615/critreveukargeneexpr.v22.i1.30. Crit Rev Eukaryot Gene Expr. 2012. PMID: 22339658 Review.
Cited by
-
Transcriptional regulation and drug resistance in Mycobacterium tuberculosis.Front Cell Infect Microbiol. 2022 Sep 2;12:990312. doi: 10.3389/fcimb.2022.990312. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36118045 Free PMC article. Review.
-
Phenotypic adaptation of Mycobacterium tuberculosis to host-associated stressors that induce persister formation.Front Cell Infect Microbiol. 2022 Sep 27;12:956607. doi: 10.3389/fcimb.2022.956607. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36237425 Free PMC article. Review.
-
Updated and standardized genome-scale reconstruction of Mycobacterium tuberculosis H37Rv, iEK1011, simulates flux states indicative of physiological conditions.BMC Syst Biol. 2018 Mar 2;12(1):25. doi: 10.1186/s12918-018-0557-y. BMC Syst Biol. 2018. PMID: 29499714 Free PMC article.
-
The biology of ergothioneine, an antioxidant nutraceutical.Nutr Res Rev. 2020 Dec;33(2):190-217. doi: 10.1017/S0954422419000301. Epub 2020 Feb 13. Nutr Res Rev. 2020. PMID: 32051057 Free PMC article. Review.
-
Host-pathogen redox dynamics modulate Mycobacterium tuberculosis pathogenesis.Pathog Dis. 2018 Jul 1;76(5):fty036. doi: 10.1093/femspd/fty036. Pathog Dis. 2018. PMID: 29873719 Free PMC article.
References
-
- Ung K. S., Av-Gay Y. (2006) Mycothiol-dependent mycobacterial response to oxidative stress. FEBS Lett. 580, 2712–2716 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

