Consistency of quality attributes for the glycosylated monoclonal antibody Humira® (adalimumab)
- PMID: 26230301
- PMCID: PMC4622832
- DOI: 10.1080/19420862.2015.1073429
Consistency of quality attributes for the glycosylated monoclonal antibody Humira® (adalimumab)
Abstract
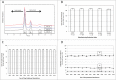
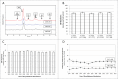
Humira® (adalimumab) is a recombinant human IgG1 monoclonal antibody (mAb) glycoprotein consisting of 1330 amino acids that is specific for human tumor necrosis factor (TNF). The biological activity and clinical profile of mAb therapeutics, including adalimumab, is influenced by their protein structure and glycosylation patterns, which can be affected by the expression system, cell culture conditions and purification process methodology. While clinical outcome cannot yet be attributed to many of the individual structural features that constitute a mAb, it is evident that detailed structural attribute analysis is necessary if structural contributions to function are to be comprehensively defined. Adalimumab product quality data generated from over a decade of manufacturing across multiple production sites and through a series of manufacturing scale changes are presented here. These data reveal a consistent and tightly controlled profile for the product.
Keywords: adalimumab; biosimilars; manufacturing; oligosaccharides; quality attributes.
Figures

References
-
- Abbvie Inc. Humira® [package insert] North Chicago, IL: 2014.
-
- Burmester GR, Panaccione R, Gordon KB, McIlraith MJ, Lacerda AP. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease. Ann Rheum Dis 2013; 72:517-24; PMID:22562972; http://dx.doi.org/10.1136/annrheumdis-2011-201244 - DOI - PMC - PubMed
-
- Gordon K, Papp K, Poulin Y, Gu Y, Rozzo S, Sasso EH. Long-term efficacy and safety of adalimumab in patients with moderate to severe psoriasis treated continuously over 3 years: results from an open-label extension study for patients from REVEAL. J Am Acad Dermatol 2012; 66:241-51; PMID:21752491; http://dx.doi.org/10.1016/j.jaad.2010.12.005 - DOI - PubMed
-
- Keystone EC, van der Heijde D, Kavanaugh A, Kupper H, Liu S, Guerette B, Mozaffarian N. Clinical, functional, and radiographic benefits of longterm adalimumab plus methotrexate: final 10-year data in longstanding rheumatoid arthritis. J Rheumatol 2013; 40:1487-97; PMID:23818718; http://dx.doi.org/10.3899/jrheum.120964 - DOI - PubMed
-
- Schiestl M, Stangler T, Torella C, Cepeljnik T, Toll H, Grau R. Acceptable changes in quality attributes of glycosylated biopharmaceuticals. Nat Biotechnol 2011; 29:310-2; PMID:21478841; http://dx.doi.org/10.1038/nbt.1839 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
