Airway Inflammation after Bronchial Thermoplasty for Severe Asthma
- PMID: 26230374
- PMCID: PMC5467092
- DOI: 10.1513/AnnalsATS.201502-082OC
Airway Inflammation after Bronchial Thermoplasty for Severe Asthma
Abstract
Rationale: Bronchial thermoplasty is an alternative treatment for patients with severe, uncontrolled asthma in which the airway smooth muscle is eliminated using radioablation. Although this emerging therapy shows promising outcomes, little is known about its effects on airway inflammation.
Objectives: We examined the presence of bronchoalveolar lavage cytokines and expression of smooth muscle actin in patients with severe asthma before and in the weeks after bronchial thermoplasty.
Methods: Endobronchial biopsies and bronchoalveolar lavage samples from 11 patients with severe asthma were collected from the right lower lobe before and 3 and 6 weeks after initial bronchial thermoplasty. Samples were analyzed for cell proportions and cytokine concentrations in bronchoalveolar lavage and for the presence of α-SMA in endobronchial biopsies.
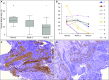
Measurements and main results: α-SMA expression was decreased in endobronchial biopsies of 7 of 11 subjects by Week 6. In bronchoalveolar lavage fluid, both transforming growth factor-β1 and regulated upon activation, normal T-cell expressed and secreted (RANTES)/CCL5 were substantially decreased 3 and 6 weeks post bronchial thermoplasty in all patients. The cytokine tumor-necrosis-factor-related apoptosis-inducing ligand (TRAIL), which induces apoptosis in several cell types, was increased in concentration both 3 and 6 weeks post bronchial thermoplasty.
Conclusions: Clinical improvement and reduction in α-SMA after bronchial thermoplasty in severe, uncontrolled asthma is associated with substantial changes in key mediators of inflammation. These data confirm the substantial elimination of airway smooth muscle post thermoplasty in the human asthmatic airway and represent the first characterization of significant changes in airway inflammation in the first weeks after thermoplasty.
Keywords: airway inflammation; airway smooth muscle; asthma; pulmonary disease.
Figures

References
-
- Barnett SB, Nurmagambetov TA. Costs of asthma in the United States: 2002-2007. J Allergy Clin Immunol. 2011;127:145–152. - PubMed
-
- Smith DH, Malone DC, Lawson KA, Okamoto LJ, Battista C, Saunders WB. A national estimate of the economic costs of asthma. Am J Respir Crit Care Med. 1997;156:787–793. - PubMed
-
- Ray A, Cohn L. Altering the Th1/Th2 balance as a therapeutic strategy in asthmatic diseases. Curr Opin Investig Drugs. 2000;1:442–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

