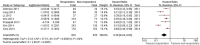Prognostic Relevance of Objective Response According to EASL Criteria and mRECIST Criteria in Hepatocellular Carcinoma Patients Treated with Loco-Regional Therapies: A Literature-Based Meta-Analysis
- PMID: 26230853
- PMCID: PMC4521926
- DOI: 10.1371/journal.pone.0133488
Prognostic Relevance of Objective Response According to EASL Criteria and mRECIST Criteria in Hepatocellular Carcinoma Patients Treated with Loco-Regional Therapies: A Literature-Based Meta-Analysis
Abstract
Background: The European Association for the Study of the Liver (EASL) criteria and the modified Response Evaluation Criteria in Solid Tumors (mRECIST) are currently adopted to evaluate radiological response in patients affected by HCC and treated with loco-regional procedures. Several studies explored the validity of these measurements in predicting survival but definitive data are still lacking.
Aim: To conduct a systematic review of studies exploring mRECIST and EASL criteria usefulness in predictive radiological response in HCC undergoing loco-regional therapies and their validity in predicting survival.
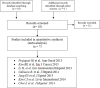
Methods: A comprehensive search of the literature was performed in electronic databases EMBASE, MEDLINE, COCHRANE LIBRARY, ASCO conferences and EASL conferences up to June 10, 2014. Our overall search strategy included terms for HCC, mRECIST, and EASL. Loco-regional procedures included transarterial embolization (TAE), transarterial chemoembolization (TACE) and cryoablation. Inter-method agreement between EASL and mRECIST was assessed using the k coefficient. For each criteria, overall survival was described in responders vs. non-responders patients, considering all target lesions response.
Results: Among 18 initially found publications, 7 reports including 1357 patients were considered eligible. All studies were published as full-text articles. Proportion of responders according to mRECIST and EASL criteria was 62.4% and 61.3%, respectively. In the pooled population, 1286 agreements were observed between the two methods (kappa statistics 0.928, 95% confidence interval 0.912-0.944). HR for overall survival (responders versus non responders) according to mRECIST and EASL was 0.39 (95% confidence interval 0.26-0.61, p<0.0001) and 0.38 (95% confidence interval 0.24-0.61, p<0.0001), respectively.
Conclusion: In this literature-based meta-analysis, mRECIST and EASL criteria showed very good concordance in HCC patients undergoing loco-regional treatments. Objective response according to both criteria confirms a strong prognostic value in terms of overall survival. This prognostic value appears to be very similar between the two criteria.
Conflict of interest statement
Figures
Similar articles
-
EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization.J Hepatol. 2011 Dec;55(6):1309-16. doi: 10.1016/j.jhep.2011.03.007. Epub 2011 Apr 15. J Hepatol. 2011. PMID: 21703196
-
Comparison of the methods for tumor response assessment in patients with hepatocellular carcinoma undergoing transarterial chemoembolization.J Hepatol. 2013 Jun;58(6):1181-7. doi: 10.1016/j.jhep.2013.01.039. Epub 2013 Feb 8. J Hepatol. 2013. PMID: 23395691
-
Prospective comparison of prognostic values of modified Response Evaluation Criteria in Solid Tumours with European Association for the Study of the Liver criteria in hepatocellular carcinoma following chemoembolisation.Eur J Cancer. 2013 Mar;49(4):826-34. doi: 10.1016/j.ejca.2012.08.022. Epub 2012 Sep 17. Eur J Cancer. 2013. PMID: 22995582
-
Evolution from WHO to EASL and mRECIST for hepatocellular carcinoma: considerations for tumor response assessment.Expert Rev Gastroenterol Hepatol. 2015 Mar;9(3):335-48. doi: 10.1586/17474124.2015.959929. Epub 2014 Nov 5. Expert Rev Gastroenterol Hepatol. 2015. PMID: 25370168 Review.
-
Assessment of treatment response in hepatocellular carcinoma: a review of the literature.Future Oncol. 2013 Jun;9(6):845-54. doi: 10.2217/fon.13.33. Future Oncol. 2013. PMID: 23718305 Review.
Cited by
-
Volumetric Analysis of Hepatocellular Carcinoma After Transarterial Chemoembolization and its Impact on Overall Survival.In Vivo. 2022 Sep-Oct;36(5):2332-2341. doi: 10.21873/invivo.12964. In Vivo. 2022. PMID: 36099102 Free PMC article.
-
Hepatic Arterial Embolization Using Cone Beam CT with Tumor Feeding Vessel Detection Software: Impact on Hepatocellular Carcinoma Response.Cardiovasc Intervent Radiol. 2018 Jan;41(1):104-111. doi: 10.1007/s00270-017-1758-2. Epub 2017 Aug 2. Cardiovasc Intervent Radiol. 2018. PMID: 28770316 Free PMC article.
-
Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma.Nat Rev Gastroenterol Hepatol. 2021 May;18(5):293-313. doi: 10.1038/s41575-020-00395-0. Epub 2021 Jan 28. Nat Rev Gastroenterol Hepatol. 2021. PMID: 33510460 Review.
-
Hepatocellular carcinoma.Nat Rev Dis Primers. 2021 Jan 21;7(1):6. doi: 10.1038/s41572-020-00240-3. Nat Rev Dis Primers. 2021. PMID: 33479224 Review.
-
The Evolving Scenario in the Assessment of Radiological Response for Hepatocellular Carcinoma in the Era of Immunotherapy: Strengths and Weaknesses of Surrogate Endpoints.Biomedicines. 2022 Nov 6;10(11):2827. doi: 10.3390/biomedicines10112827. Biomedicines. 2022. PMID: 36359347 Free PMC article. Review.
References
-
- Parkin DM, et al. Global cancer statistics, 2002. CA Cancer J Clin, 2005. 55(2): p. 74–108. - PubMed
-
- Fattovich G., et al., Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology, 2004. 127(5 Suppl 1): p. S35–50. - PubMed
-
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet, 2003. 362(9399): p. 1907–17. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous