Protein Tyrosine Phosphatase PTPRS Is an Inhibitory Receptor on Human and Murine Plasmacytoid Dendritic Cells
- PMID: 26231120
- PMCID: PMC4547994
- DOI: 10.1016/j.immuni.2015.07.009
Protein Tyrosine Phosphatase PTPRS Is an Inhibitory Receptor on Human and Murine Plasmacytoid Dendritic Cells
Abstract
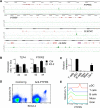
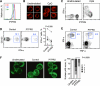
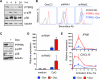
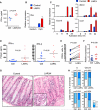
Plasmacytoid dendritic cells (pDCs) are primary producers of type I interferon (IFN) in response to viruses. The IFN-producing capacity of pDCs is regulated by specific inhibitory receptors, yet none of the known receptors are conserved in evolution. We report that within the human immune system, receptor protein tyrosine phosphatase sigma (PTPRS) is expressed specifically on pDCs. Surface PTPRS was rapidly downregulated after pDC activation, and only PTPRS(-) pDCs produced IFN-α. Antibody-mediated PTPRS crosslinking inhibited pDC activation, whereas PTPRS knockdown enhanced IFN response in a pDC cell line. Similarly, murine Ptprs and the homologous receptor phosphatase Ptprf were specifically co-expressed in murine pDCs. Haplodeficiency or DC-specific deletion of Ptprs on Ptprf-deficient background were associated with enhanced IFN response of pDCs, leukocyte infiltration in the intestine and mild colitis. Thus, PTPRS represents an evolutionarily conserved pDC-specific inhibitory receptor, and is required to prevent spontaneous IFN production and immune-mediated intestinal inflammation.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures



References
-
- Bar-On L, Zigmond E, Jung S. Management of gut inflammation through the manipulation of intestinal dendritic cells and macrophages? Semin. Immunol. 2011;23:58–64. - PubMed
-
- Blasius A, Vermi W, Krug A, Facchetti F, Cella M, Colonna M. A cell-surface molecule selectively expressed on murine natural inter-feron-producing cells that blocks secretion of interferon-alpha. Blood. 2004;103:4201–4206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

