Ligand Field Strength Mediates Electron Delocalization in Octahedral [((H)L)2Fe6(L')m](n+) Clusters
- PMID: 26231520
- PMCID: PMC5572642
- DOI: 10.1021/jacs.5b06453
Ligand Field Strength Mediates Electron Delocalization in Octahedral [((H)L)2Fe6(L')m](n+) Clusters
Abstract
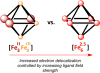
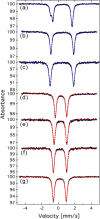
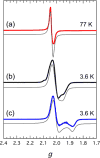
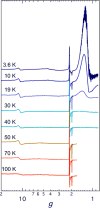
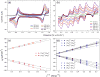
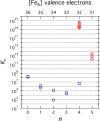
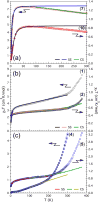
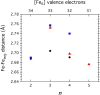
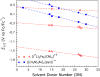
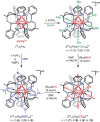
To assess the impact of terminal ligand binding on a variety of cluster properties (redox delocalization, ground-state stabilization, and breadth of redox state accessibility), we prepared three electron-transfer series based on the hexanuclear iron cluster [((H)L)2Fe6(L')m](n+) in which the terminal ligand field strength was modulated from weak to strong (L' = DMF, MeCN, CN). The extent of intracore M-M interactions is gauged by M-M distances, spin ground state persistence, and preference for mixed-valence states as determined by electrochemical comproportionation constants. Coordination of DMF to the [((H)L)2Fe6] core leads to weaker Fe-Fe interactions, as manifested by the observation of ground states populated only at lower temperatures (<100 K) and by the greater evidence of valence trapping within the mixed-valence states. Comproportionation constants determined electrochemically (Kc = 10(4)-10(8)) indicate that the redox series exhibits electronic delocalization (class II-III), yet no intervalence charge transfer (IVCT) bands are observable in the near-IR spectra. Ligation of the stronger σ donor acetonitrile results in stabilization of spin ground states to higher temperatures (∼300 K) and a high degree of valence delocalization (Kc = 10(2)-10(8)) with observable IVCT bands. Finally, the anionic cyanide-bound series reveals the highest degree of valence delocalization with the most intense IVCT bands (Kc = 10(12)-10(20)) and spin ground state population beyond room temperature. Across the series, at a given formal oxidation level, the capping ligand on the hexairon cluster dictates the overall properties of the aggregate, modulating the redox delocalization and the persistence of the intracore coupling of the metal sites.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
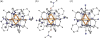



Similar articles
-
Thermally Persistent High-Spin Ground States in Octahedral Iron Clusters.J Am Chem Soc. 2018 Dec 5;140(48):16792-16806. doi: 10.1021/jacs.8b10181. Epub 2018 Nov 21. J Am Chem Soc. 2018. PMID: 30403845
-
Electronic structure of linear thiophenolate-bridged heteronuclear complexes [LFeMFeL](n)(+) (M = Cr, Co, Fe; n = 1-3): a combination of kinetic exchange interaction and electron delocalization.J Am Chem Soc. 2003 Oct 15;125(41):12615-30. doi: 10.1021/ja030027t. J Am Chem Soc. 2003. PMID: 14531706
-
Structural and spectroscopic studies of valence-delocalized diiron(II,III) complexes dupported by carboxylate-only bridging ligands.Inorg Chem. 2002 Jun 17;41(12):3172-82. doi: 10.1021/ic011050n. Inorg Chem. 2002. PMID: 12054996
-
[((H)L)2Fe6(NCMe)m]n+ (m = 0, 2, 4, 6; n = -1, 0, 1, 2, 3, 4, 6): an electron-transfer series featuring octahedral Fe6 clusters supported by a hexaamide ligand platform.J Am Chem Soc. 2011 Jun 1;133(21):8293-306. doi: 10.1021/ja2015845. Epub 2011 May 11. J Am Chem Soc. 2011. PMID: 21561083
-
Mixed valency in ligand-bridged diruthenium frameworks: divergences and perspectives.RSC Adv. 2018 Aug 14;8(51):28895-28908. doi: 10.1039/c8ra03206h. eCollection 2018 Aug 14. RSC Adv. 2018. PMID: 35547993 Free PMC article. Review.
Cited by
-
Harnessing Electrostatic Interactions for Enhanced Conductivity in Metal-Organic Frameworks.Research (Wash D C). 2021 Oct 21;2021:9874273. doi: 10.34133/2021/9874273. eCollection 2021. Research (Wash D C). 2021. PMID: 34778792 Free PMC article.
-
Meta-Atom Behavior in Clusters Revealing Large Spin Ground States.J Am Chem Soc. 2015 Nov 4;137(43):13949-56. doi: 10.1021/jacs.5b08962. Epub 2015 Oct 21. J Am Chem Soc. 2015. PMID: 26440452 Free PMC article.
-
Exposing the inadequacy of redox formalisms by resolving redox inequivalence within isovalent clusters.Proc Natl Acad Sci U S A. 2019 Aug 6;116(32):15836-15841. doi: 10.1073/pnas.1907699116. Epub 2019 Jul 19. Proc Natl Acad Sci U S A. 2019. PMID: 31324742 Free PMC article.
-
Nitrogen Fixation via a Terminal Fe(IV) Nitride.J Am Chem Soc. 2017 Nov 1;139(43):15312-15315. doi: 10.1021/jacs.7b09364. Epub 2017 Oct 19. J Am Chem Soc. 2017. PMID: 28992418 Free PMC article.
-
An iron ketimide single-molecule magnet [Fe4(N[double bond, length as m-dash]CPh2)6] with suppressed through-barrier relaxation.Chem Sci. 2020 Apr 20;11(18):4753-4757. doi: 10.1039/d0sc01578d. Chem Sci. 2020. PMID: 34122931 Free PMC article.
References
-
- Day P, Hush NS, Clark RJH. Philos Trans R Soc, A. 2008;366:5. - PubMed
-
- Hagen WR, Wassink H, Eady RR, Smith BE, Haaker H. Eur J Biochem. 1987;169:457. - PubMed
- Pierik AJ, Hagen WR. Eur J Biochem. 1991;195:505. - PubMed
- Pierik AJ, Hagen WR, Dunham WR, Sands RH. Eur J Biochem. 1992;206:705. - PubMed
- Pierik AJ, Wassink H, Haaker H, Hagen WR. Eur J Biochem. 1993;212:51. - PubMed
-
- Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S. Science. 2004;303:1831. - PubMed
- Kanan MW, Nocera DG. Science. 2008;321:1072. - PubMed
- Wang D, Farquhar ER, Stubna A, Munck E, Que L. Nat Chem. 2009;1:145. - PMC - PubMed
- Lee CC, Hu YL, Ribbe MW. Science. 2010;329:642. - PMC - PubMed
- Xue GQ, De Hont R, Munck E, Que L. Nat Chem. 2010;2:400. - PMC - PubMed
- Surendranath Y, Kanan MW, Nocera DG. J Am Chem Soc. 2010;132:16501. - PubMed
- Hu YL, Lee CC, Ribbe MW. Science. 2011;333:753. - PMC - PubMed
- Reece SY, Hamel JA, Sung K, Jarvi TD, Esswein AJ, Pijpers JJH, Nocera DG. Science. 2011;334:645. - PubMed
- Bediako DK, Surendranath Y, Nocera DG. J Am Chem Soc. 2013;135:3662. - PubMed
- Morrison CN, Hoy JA, Zhang LM, Einsle O, Rees DC. Biochemistry. 2015;54:2052. - PMC - PubMed
-
- Kaim W, Klein A, Glockle M. Acc Chem Res. 2000;33:755. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

