Fluorescent proteins function as a prey attractant: experimental evidence from the hydromedusa Olindias formosus and other marine organisms
- PMID: 26231627
- PMCID: PMC4582119
- DOI: 10.1242/bio.012138
Fluorescent proteins function as a prey attractant: experimental evidence from the hydromedusa Olindias formosus and other marine organisms
Abstract
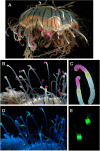
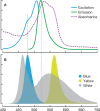
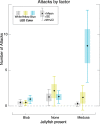
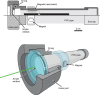
Although proteins in the green fluorescent protein family (GFPs) have been discovered in a wide array of taxa, their ecological functions in these organisms remain unclear. Many hypothesized roles are related to modifying bioluminescence spectra or modulating the light regime for algal symbionts, but these do not explain the presence of GFPs in animals that are non-luminous and non-symbiotic. Other hypothesized functions are unrelated to the visual signals themselves, including stress responses and antioxidant roles, but these cannot explain the localization of fluorescence in particular structures on the animals. Here we tested the hypothesis that fluorescence might serve to attract prey. In laboratory experiments, the predator was the hydromedusa Olindias formosus (previously known as O. formosa), which has fluorescent and pigmented patches on the tips of its tentacles. The prey, juvenile rockfishes in the genus Sebastes, were significantly more attracted (P<1×10(-5)) to the medusa's tentacles under lighting conditions where fluorescence was excited and tentacle tips were visible above the background. The fish did not respond significantly when treatments did not include fluorescent structures or took place under yellow or white lights, which did not generate fluorescence visible above the ambient light. Furthermore, underwater observations of the behavior of fishes when presented with a brightly illuminated point showed a strong attraction to this visual stimulus. In situ observations also provided evidence for fluorescent lures as supernormal stimuli in several other marine animals, including the siphonophore Rhizophysa eysenhardti. Our results support the idea that fluorescent structures can serve as prey attractants, thus providing a potential function for GFPs and other fluorescent proteins in a diverse range of organisms.
Keywords: Feeding behavior; Fluorescent protein; GFP; Olindias; Prey attraction; Supernormal stimulus.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures



References
-
- Britt L. L., Loew E. R. and McFarland W. N. (2001). Visual pigments in the early life stages of Pacific Northwest marine fishes. J. Exp. Biol. 204, 2581-2587. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

