Publication Speed, Reporting Metrics, and Citation Impact of Cardiovascular Trials Supported by the National Heart, Lung, and Blood Institute
- PMID: 26231845
- PMCID: PMC4599480
- DOI: 10.1161/JAHA.115.002292
Publication Speed, Reporting Metrics, and Citation Impact of Cardiovascular Trials Supported by the National Heart, Lung, and Blood Institute
Abstract
Background: We previously demonstrated that cardiovascular (CV) trials funded by the National Heart, Lung, and Blood Institute (NHLBI) were more likely to be published in a timely manner and receive high raw citation counts if they focused on clinical endpoints. We did not examine the metrics of trial reports, and our citation measures were limited by failure to account for topic-related citation behaviors.
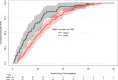
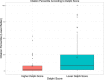
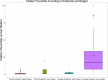
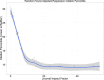
Methods and results: Of 244 CV trials completed between 2000 and 2011, we identified 184 whose main results were published by August 20, 2014. One investigator who was blinded to rapidity of publication and citation data read each publication and characterized it according to modified Delphi criteria. There were 46 trials (25%) that had Delphi scores of 8 or 9 (of a possible 9); these trials published faster (median time from trial completion to publication, 12.6 [interquartile range {IQR}, 6.7 to 23.3] vs. 21.8 [IQR, 12.1 to 34.9] months; P<0.01). They also had better normalized citation impact (median citation percentile for topic and date of publication, with 0 best and 100 worst, 1.92 [IQR, 0.64 to 7.83] vs. 8.41 [IQR, 1.80 to 24.75]; P=0.002). By random forest regression, we found that the 3 most important predictors of normalized citation percentile values were total costs, intention-to-treat analyses (as a modified Delphi quality measure), and focus on clinical (not surrogate) endpoints.
Conclusions: NHLBI CV trials were more likely to publish results quickly and yield higher topic-normalized citation impact if they reported results according to well-defined metrics, along with focus on clinical endpoints.
Keywords: bibliometrics; citation; public policy; randomized, controlled trial; research funding.
© 2015 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures


Similar articles
-
Publication of trials funded by the National Heart, Lung, and Blood Institute.N Engl J Med. 2013 Nov 14;369(20):1926-34. doi: 10.1056/NEJMsa1300237. N Engl J Med. 2013. PMID: 24224625 Free PMC article.
-
Percentile ranking and citation impact of a large cohort of National Heart, Lung, and Blood Institute-funded cardiovascular R01 grants.Circ Res. 2014 Feb 14;114(4):600-6. doi: 10.1161/CIRCRESAHA.114.302656. Epub 2014 Jan 9. Circ Res. 2014. PMID: 24406983 Free PMC article.
-
Differential citation rates of major cardiovascular clinical trials according to source of funding: a survey from 2000 to 2005.Circulation. 2008 Sep 23;118(13):1321-7. doi: 10.1161/CIRCULATIONAHA.108.794016. Epub 2008 Sep 8. Circulation. 2008. PMID: 18779441
-
Determinants of Citation Impact in Large Clinical Trials in Critical Care: The Role of Investigator-Led Clinical Trials Groups.Crit Care Med. 2016 Apr;44(4):663-70. doi: 10.1097/CCM.0000000000001466. Crit Care Med. 2016. PMID: 26571189 Review.
-
Predictors of citations in the urological literature.BJU Int. 2011 Jun;107(12):1876-80. doi: 10.1111/j.1464-410X.2010.10028.x. Epub 2011 Feb 18. BJU Int. 2011. PMID: 21332629 Review.
Cited by
-
Analysis of publication speed of anesthesiology journals: a cross-sectional study.Braz J Anesthesiol. 2021 Mar-Apr;71(2):110-115. doi: 10.1016/j.bjane.2021.02.025. Epub 2021 Feb 19. Braz J Anesthesiol. 2021. PMID: 33731261 Free PMC article.
-
Efficient design of clinical trials and epidemiological research: is it possible?Nat Rev Cardiol. 2017 Aug;14(8):493-501. doi: 10.1038/nrcardio.2017.60. Epub 2017 Apr 27. Nat Rev Cardiol. 2017. PMID: 28447664 Review.
-
Publication speed in pharmacy practice journals: A comparative analysis.PLoS One. 2021 Jun 29;16(6):e0253713. doi: 10.1371/journal.pone.0253713. eCollection 2021. PLoS One. 2021. PMID: 34185802 Free PMC article.
-
Design and implementation of the Resuscitation Outcomes Consortium Pragmatic Airway Resuscitation Trial (PART).Resuscitation. 2016 Apr;101:57-64. doi: 10.1016/j.resuscitation.2016.01.012. Epub 2016 Feb 2. Resuscitation. 2016. PMID: 26851059 Free PMC article. Clinical Trial.
-
Journal impact factor, trial effect size, and methodological quality appear scantly related: a systematic review and meta-analysis.Syst Rev. 2020 Mar 9;9(1):53. doi: 10.1186/s13643-020-01305-w. Syst Rev. 2020. PMID: 32164791 Free PMC article.
References
-
- Devereaux PJ, Yusuf S. When it comes to trials, do we get what we pay for? N Engl J Med. 2013;369:1962–1963. - PubMed
-
- Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, Knipschild PG. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51:1235–1241. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

