Efficacy and safety of LCZ696 (sacubitril-valsartan) according to age: insights from PARADIGM-HF
- PMID: 26231885
- PMCID: PMC4595742
- DOI: 10.1093/eurheartj/ehv330
Efficacy and safety of LCZ696 (sacubitril-valsartan) according to age: insights from PARADIGM-HF
Abstract
Background: The age at which heart failure develops varies widely between countries and drug tolerance and outcomes also vary by age. We have examined the efficacy and safety of LCZ696 according to age in the Prospective comparison of angiotensin receptor neprilysin inhibitor with angiotensin converting enzyme inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure trial (PARADIGM-HF).
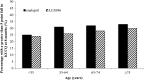
Methods: In PARADIGM-HF, 8399 patients aged 18-96 years and in New York Heart Association functional class II-IV with an LVEF ≤40% were randomized to either enalapril or LCZ696. We examined the pre-specified efficacy and safety outcomes according to age category (years): <55 (n = 1624), 55-64 (n = 2655), 65-74 (n = 2557), and ≥75 (n = 1563).
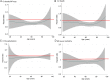
Findings: The rate (per 100 patient-years) of the primary outcome of cardiovascular (CV) death or heart failure hospitalization (HFH) increased from 13.4 to 14.8 across the age categories. The LCZ696:enalapril hazard ratio (HR) was <1.0 in all categories (P for interaction between age category and treatment = 0.94) with an overall HR of 0.80 (0.73, 0.87), P < 0.001. The findings for HFH were similar for CV and all-cause mortality and the age category by treatment interactions were not significant. The pre-specified safety outcomes of hypotension, renal impairment and hyperkalaemia increased in both treatment groups with age, although the differences between treatment (more hypotension but less renal impairment and hyperkalaemia with LCZ696) were consistent across age categories.
Interpretation: LCZ696 was more beneficial than enalapril across the spectrum of age in PARADIGM-HF with a favourable benefit-risk profile in all age groups.
Trial registration: ClinicalTrials.gov NCT01035255.
Keywords: Age; Angiotensin; Heart failure; Neprilysin; Receptors.
© The Author 2015. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures

References
-
- Callender T, Woodward M, Roth G, Farzadfar F, Lemarie JC, Gicquel S, Atherton J, Rahimzadeh S, Ghaziani M, Shaikh M, Bennett D, Patel A, Lam CS, Sliwa K, Barretto A, Siswanto BB, Diaz A, Herpin D, Krum H, Eliasz T, Forbes A, Kiszely A, Khosla R, Petrinic T, Praveen D, Shrivastava R, Xin D, MacMahon S, McMurray J, Rahimi K. Heart failure care in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med 2014;11:e1001699. - PMC - PubMed
-
- Maggioni AP, Dahlström U, Filippatos G, Chioncel O, Leiro MC, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors A, Nielsen OW, Zannad F, Tavazzi L, Heart Failure Association of ESC (HFA). EURObservational Research Programme: the heart failure pilot survey (ESC-HF pilot). Eur J Heart Fail 2010;12:1076–1084. - PubMed
-
- Krim SR, Vivo RP, Krim NR, Qian F, Cox M, Ventura H, Hernandez AF, Bhatt DL, Fonarow GC. Racial/Ethnic differences in B-type natriuretic peptide levels and their association with care and outcomes among patients hospitalized with heart failure: findings from Get with the Guidelines-Heart Failure. JACC Heart Fail 2013;1:345–352. - PubMed
-
- McAlister FA, Bakal JA, Kaul P, Quan H, Blackadar R, Johnstone D, Ezekowitz J. Changes in heart failure outcomes after a province-wide change in health service provision a natural experiment in Alberta, Canada. Circ Heart Fail 2013;6:76–82. - PubMed
-
- McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau J, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM-HF Committees and Investigators. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail 2013;15:1062–1073. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

