Pilot study of sphenopalatine injection of onabotulinumtoxinA for the treatment of intractable chronic cluster headache
- PMID: 26232105
- PMCID: PMC4853809
- DOI: 10.1177/0333102415597891
Pilot study of sphenopalatine injection of onabotulinumtoxinA for the treatment of intractable chronic cluster headache
Abstract
Objective: The main object of this pilot study was to investigate the safety of administering onabotulinumtoxinA (BTA) towards the sphenopalatine ganglion (SPG) in intractable chronic cluster headache. Efficacy data were also collected to provide indication on whether future placebo-controlled studies should be performed.
Method: In a prospective, open-label, uncontrolled study, we performed a single injection of 25 IU (n = 5) or 50 IU BTA (n = 5) towards the SPG in 10 patients with intractable chronic cluster headache with a follow-up of 24 weeks. The primary outcome was adverse events (AEs) and the main efficacy outcome was attack frequency in weeks 3 and 4 post-treatment.
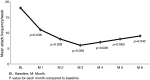
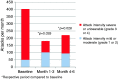
Results: A total of 11 AEs were registered. There was one severe adverse event (SAE): posterior epistaxis. The number of cluster headache attacks (main efficacy outcome) was statistically significantly reduced in the intention-to-treat analysis from 18 ± 12 per week in baseline to 11 ± 14 (p = 0.038) in weeks 3 and 4, and five out of 10 patients had at least 50% reduction of attack frequency compared to baseline. The cluster attack frequency was significantly reduced for five out of six months post-treatment.
Conclusion: Randomised, placebo-controlled studies are warranted to establish the potential of this possible novel treatment of cluster headache.
Keywords: Cluster headache; botulinum toxin; headache; pterygopalatine ganglion; sphenopalatine ganglion.
© International Headache Society 2015.
Figures
Similar articles
-
Open-Label, Multi-Dose, Pilot Safety Study of Injection of OnabotulinumtoxinA Toward the Otic Ganglion for the Treatment of Intractable Chronic Cluster Headache.Headache. 2020 Sep;60(8):1632-1643. doi: 10.1111/head.13889. Epub 2020 Jun 25. Headache. 2020. PMID: 32583902 Clinical Trial.
-
Pilot study of sphenopalatine injection of onabotulinumtoxinA for the treatment of intractable chronic migraine.Cephalalgia. 2017 Apr;37(4):356-364. doi: 10.1177/0333102416648328. Epub 2016 May 6. Cephalalgia. 2017. PMID: 27154997 Free PMC article. Clinical Trial.
-
Long-Term Outcome of Patients With Intractable Chronic Cluster Headache Treated With Injection of Onabotulinum Toxin A Toward the Sphenopalatine Ganglion - An Observational Study.Headache. 2018 Nov;58(10):1519-1529. doi: 10.1111/head.13398. Epub 2018 Sep 14. Headache. 2018. PMID: 30216444 Free PMC article.
-
Sphenopalatine ganglion stimulation in cluster headache and other types of headache.Cephalalgia. 2016 Oct;36(12):1149-1155. doi: 10.1177/0333102416644968. Epub 2016 Jul 11. Cephalalgia. 2016. PMID: 27152017 Review.
-
The Efficacy of Botulinum Toxin in Cluster Headache: A Systematic Review.J Oral Facial Pain Headache. 2020 Spring;34(2):129–134. doi: 10.11607/ofph.2444. Epub 2019 Sep 27. J Oral Facial Pain Headache. 2020. PMID: 31560734
Cited by
-
Expression of messenger molecules and receptors in rat and human sphenopalatine ganglion indicating therapeutic targets.J Headache Pain. 2016 Dec;17(1):78. doi: 10.1186/s10194-016-0664-3. Epub 2016 Sep 1. J Headache Pain. 2016. PMID: 27587062 Free PMC article.
-
Measurement and implications of the distance between the sphenopalatine ganglion and nasal mucosa: a neuroimaging study.J Headache Pain. 2018 Feb 13;19(1):14. doi: 10.1186/s10194-018-0843-5. J Headache Pain. 2018. PMID: 29442191 Free PMC article.
-
[Clinical use of botulinum toxin type A in pain medicine].Schmerz. 2023 Aug;37(4):297-307. doi: 10.1007/s00482-023-00730-9. Epub 2023 Jun 26. Schmerz. 2023. PMID: 37365293 German.
-
Ganglion blocks as a treatment of pain: current perspectives.J Pain Res. 2017 Dec 14;10:2815-2826. doi: 10.2147/JPR.S134775. eCollection 2017. J Pain Res. 2017. PMID: 29276402 Free PMC article. Review.
-
Onabotulinum toxin A in the treatment of chronic migraine: patient selection and special considerations.J Pain Res. 2017 Sep 29;10:2319-2329. doi: 10.2147/JPR.S113614. eCollection 2017. J Pain Res. 2017. PMID: 29033605 Free PMC article. Review.
References
-
- May A, Goadsby PJ. The trigeminovascular system in humans: Pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. J Cereb Blood Flow Metab 1999; 19: 115–127. - PubMed
-
- Sluder G. The role of the sphenopalatine ganglion in nasal headaches. N Y State J Med 1908; 27: 8–13.
-
- Prasanna A, Murthy PS. Sphenopalatine ganglion block under vision using rigid nasal sinuscope. Reg Anesth 1993; 18: 139–140. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

