Colony-stimulating factor 1 receptor inhibition prevents microglial plaque association and improves cognition in 3xTg-AD mice
- PMID: 26232154
- PMCID: PMC4522109
- DOI: 10.1186/s12974-015-0366-9
Colony-stimulating factor 1 receptor inhibition prevents microglial plaque association and improves cognition in 3xTg-AD mice
Abstract
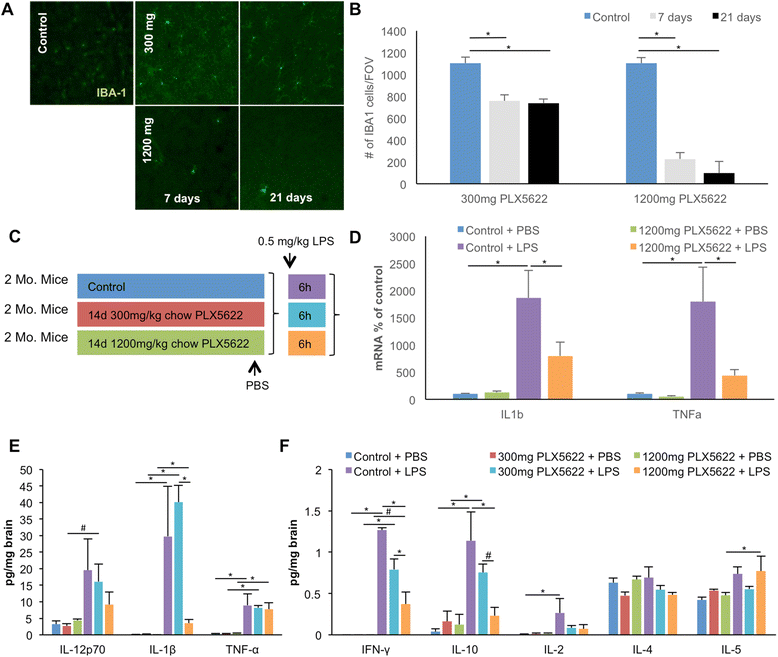
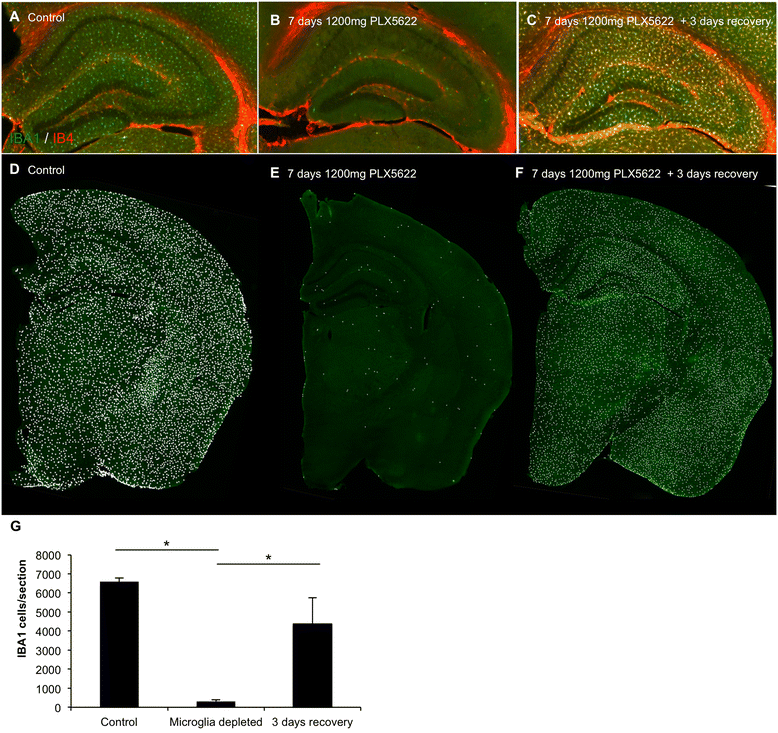
Background: Microglia are dependent upon colony-stimulating factor 1 receptor (CSF1R) signaling for their survival in the adult brain, with administration of the dual CSF1R/c-kit inhibitor PLX3397 leading to the near-complete elimination of all microglia brainwide. Here, we determined the dose-dependent effects of a specific CSF1R inhibitor (PLX5622) on microglia in both wild-type and the 3xTg-AD mouse model of Alzheimer's disease.
Methods: Wild-type mice were treated with PLX5622 for up to 21 days, and the effects on microglial numbers were assessed. 3xTg-AD mice were treated with PLX5622 for 6 or 12 weeks and effects on microglial numbers and pathology subsequently assessed.
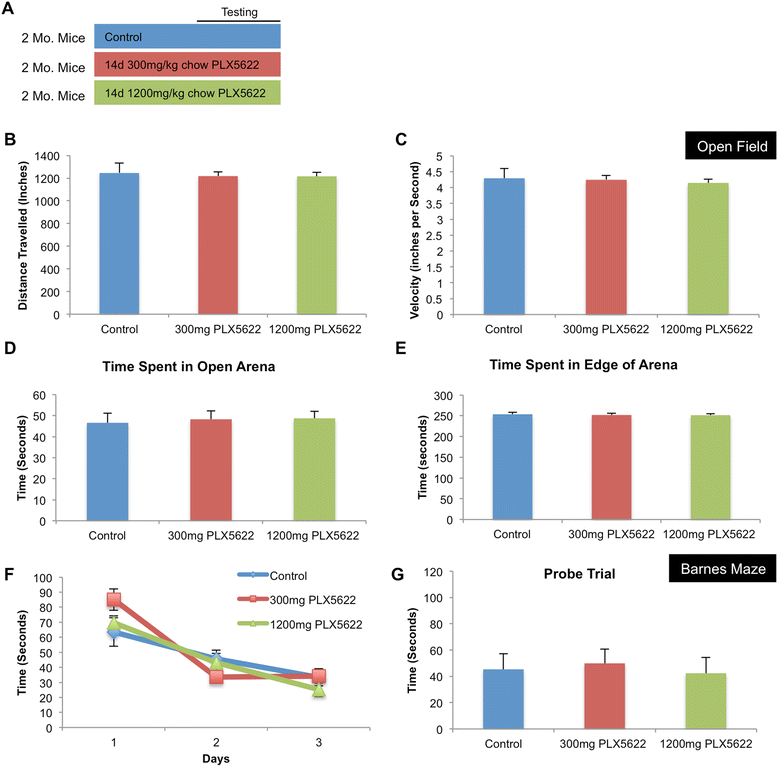
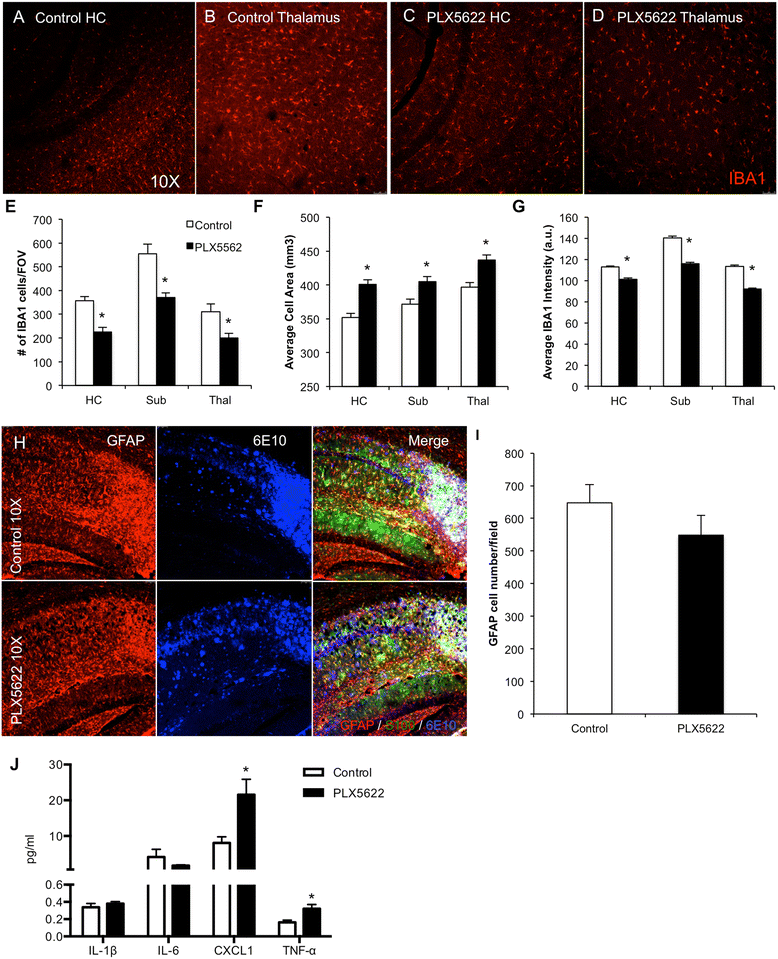
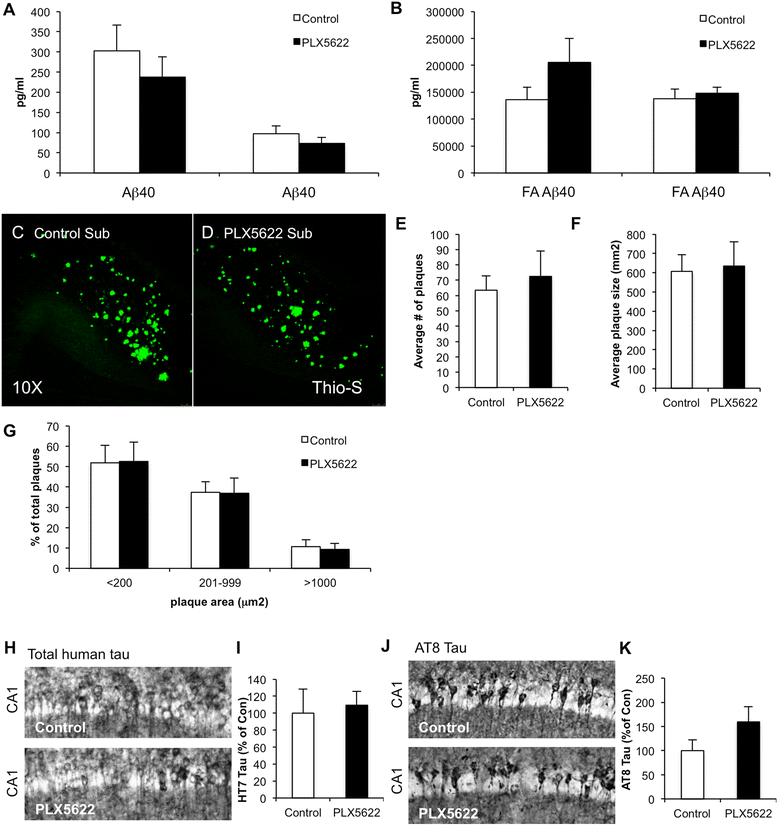
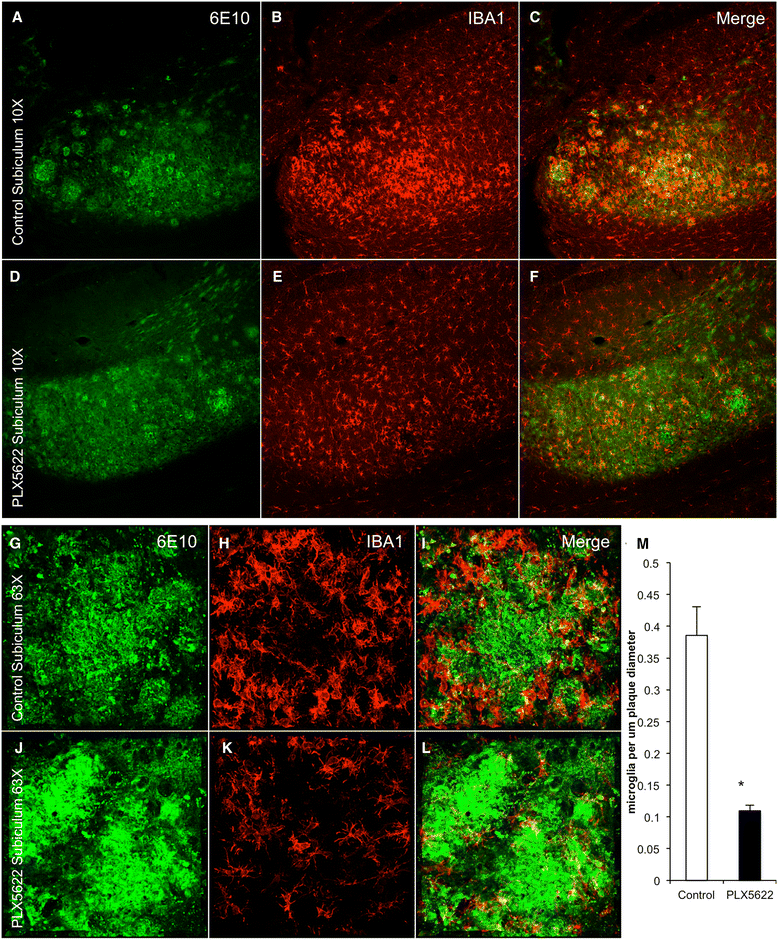
Results: High doses of CSF1R inhibitor eliminate most microglia from the brain, but a 75% lower-dose results in sustained elimination of ~30 of microglia in both wild-type and 3xTg-AD mice. No behavioral or cognitive deficits were found in mice either depleted of microglia or treated with lower CSF1R inhibitor concentrations. Aged 3xTg-AD mice treated for 6 or 12 weeks with lower levels of PLX5622 resulted in improved learning and memory. Aβ levels and plaque loads were not altered, but microglia in treated mice no longer associated with plaques, revealing a role for the CSF1R in the microglial reaction to plaques, as well as in mediating cognitive deficits.
Conclusions: We find that inhibition of CSF1R alone is sufficient to eliminate microglia and that sustained microglial elimination is concentration-dependent. Inhibition of the CSF1R at lower levels in 3xTg-AD mice prevents microglial association with plaques and improves cognition.
Figures


References
-
- Lenzlinger PM, Morganti-Kossmann MC, Laurer HL, McIntosh TK. The duality of the inflammatory response to traumatic brain injury. Mol Neurobiol. 2001;24(1–3):169–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

