Platelets induce apoptosis via membrane-bound FasL
- PMID: 26232171
- PMCID: PMC4573871
- DOI: 10.1182/blood-2013-12-544445
Platelets induce apoptosis via membrane-bound FasL
Erratum in
- Blood. 2016 Jan 14;127(2):270
-
Erratum: Schleicher RI, Reichenbach F, Kraft P, et al. Platelets induce apoptosis via membrane-bound FasL. Blood. 2015;126(12):1483-1493.Blood. 2016 Jan 14;127(2):270. doi: 10.1182/blood-2015-11-682914. Blood. 2016. PMID: 31265494 Free PMC article.
Abstract
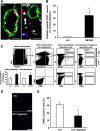
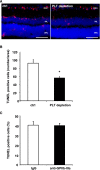
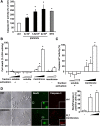
After tissue injury, both wound sealing and apoptosis contribute to restoration of tissue integrity and functionality. Although the role of platelets (PLTs) for wound closure and induction of regenerative processes is well established, the knowledge about their contribution to apoptosis is incomplete. Here, we show that PLTs present the death receptor Fas ligand (FasL) on their surface after activation. Activated PLTs as well as the isolated membrane fraction of activated PLTs but not of resting PLTs induced apoptosis in a dose-dependent manner in primary murine neuronal cells, human neuroblastoma cells, and mouse embryonic fibroblasts. Membrane protein from PLTs lacking membrane-bound FasL (FasL(△m/△m)) failed to induce apoptosis. Bax/Bak-mediated mitochondrial apoptosis signaling in target cells was not required for PLT-induced cell death, but increased the apoptotic response to PLT-induced Fas signaling. In vivo, PLT depletion significantly reduced apoptosis in a stroke model and an inflammation-independent model of N-methyl-d-aspartic acid-induced retinal apoptosis. Furthermore, experiments using PLT-specific PF4Cre(+) FasL(fl/fl) mice demonstrated a role of PLT-derived FasL for tissue apoptosis. Because apoptosis secondary to injury prevents inflammation, our findings describe a novel mechanism on how PLTs contribute to tissue homeostasis.
Figures



Similar articles
-
Fas/FasL pathway participates in resolution of mucosal inflammatory response early during HSV-2 infection.Immunobiology. 2014 Jan;219(1):64-77. doi: 10.1016/j.imbio.2013.08.002. Epub 2013 Aug 15. Immunobiology. 2014. PMID: 24028839
-
Genetically engineered blood pharming: generation of HLA-universal platelets derived from CD34+ progenitor cells.J Stem Cells. 2014;9(3):149-61. J Stem Cells. 2014. PMID: 25157449 Review.
-
Vulnerability of mouse cortical neurons to doxorubicin-induced apoptosis is strain-dependent and is correlated with mRNAs encoding Fas, Fas-Ligand, and metalloproteinases.Apoptosis. 2004 Sep;9(5):649-56. doi: 10.1023/B:APPT.0000038038.42809.e2. Apoptosis. 2004. PMID: 15314293
-
Cadmium-induced apoptosis in neuronal cells is mediated by Fas/FasL-mediated mitochondrial apoptotic signaling pathway.Sci Rep. 2018 Jun 11;8(1):8837. doi: 10.1038/s41598-018-27106-9. Sci Rep. 2018. PMID: 29891925 Free PMC article.
-
Immune privilege or inflammation? The paradoxical effects of Fas ligand.Arch Immunol Ther Exp (Warsz). 2000;48(2):73-9. Arch Immunol Ther Exp (Warsz). 2000. PMID: 10807046 Review.
Cited by
-
Cladribine treatment improves cortical network functionality in a mouse model of autoimmune encephalomyelitis.J Neuroinflammation. 2022 Nov 8;19(1):270. doi: 10.1186/s12974-022-02588-7. J Neuroinflammation. 2022. PMID: 36348455 Free PMC article.
-
Fibrinogen Level Combined With Platelet Count for Predicting Hemorrhagic Transformation in Acute Ischemic Stroke Patients Treated With Mechanical Thrombectomy.Front Neurol. 2021 Aug 31;12:716020. doi: 10.3389/fneur.2021.716020. eCollection 2021. Front Neurol. 2021. PMID: 34531815 Free PMC article.
-
Platelet Derivatives and the Immunomodulation of Wound Healing.Int J Mol Sci. 2022 Jul 28;23(15):8370. doi: 10.3390/ijms23158370. Int J Mol Sci. 2022. PMID: 35955503 Free PMC article. Review.
-
The Platelet Lifeline to Cancer: Challenges and Opportunities.Cancer Cell. 2018 Jun 11;33(6):965-983. doi: 10.1016/j.ccell.2018.03.002. Epub 2018 Apr 12. Cancer Cell. 2018. PMID: 29657130 Free PMC article. Review.
-
Platelets regulate ischemia-induced revascularization and angiogenesis by secretion of growth factor-modulating factors.Blood Adv. 2023 Nov 14;7(21):6411-6427. doi: 10.1182/bloodadvances.2021006891. Blood Adv. 2023. PMID: 37257194 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

