A multicenter randomized controlled trial of intravenous magnesium for sickle cell pain crisis in children
- PMID: 26232172
- PMCID: PMC4591790
- DOI: 10.1182/blood-2015-05-647107
A multicenter randomized controlled trial of intravenous magnesium for sickle cell pain crisis in children
Abstract
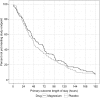
Magnesium, a vasodilator, anti-inflammatory, and pain reliever, could alter the pathophysiology of sickle cell pain crises. We hypothesized that intravenous magnesium would shorten length of stay, decrease opioid use, and improve health-related quality of life (HRQL) for pediatric patients hospitalized with sickle cell pain crises. The Magnesium for Children in Crisis (MAGiC) study was a randomized, double-blind, placebo-controlled trial of intravenous magnesium vs normal saline placebo conducted at 8 sites within the Pediatric Emergency Care Applied Research Network (PECARN). Children 4 to 21 years old with hemoglobin SS or Sβ(0) thalassemia requiring hospitalization for pain were eligible. Children received 40 mg/kg of magnesium or placebo every 8 hours for up to 6 doses plus standard therapy. The primary outcome was length of stay in hours from the time of first study drug infusion, compared using a Van Elteren test. Secondary outcomes included opioid use and HRQL. Of 208 children enrolled, 204 received the study drug (101 magnesium, 103 placebo). Between-group demographics and prerandomization treatment were similar. The median interquartile range (IQR) length of stay was 56.0 (27.0-109.0) hours for magnesium vs 47.0 (24.0-99.0) hours for placebo (P = .24). Magnesium patients received 1.46 mg/kg morphine equivalents vs 1.28 mg/kg for placebo (P = .12). Changes in HRQL before discharge and 1 week after discharge were similar (P > .05 for all comparisons). The addition of intravenous magnesium did not shorten length of stay, reduce opioid use, or improve quality of life in children hospitalized for sickle cell pain crisis. This trial was registered at www.clinicaltrials.gov as #NCT01197417.
© 2015 by The American Society of Hematology.
Figures
Comment in
-
MAGiC: VOC remains but kids with SCA appear.Blood. 2015 Oct 1;126(14):1637-8. doi: 10.1182/blood-2015-08-662502. Blood. 2015. PMID: 26429963
Similar articles
-
Intravenous magnesium for pediatric sickle cell vaso-occlusive crisis: methodological issues of a randomized controlled trial.Pediatr Blood Cancer. 2014 Jun;61(6):1049-54. doi: 10.1002/pbc.24925. Epub 2014 Jan 17. Pediatr Blood Cancer. 2014. PMID: 24443249 Free PMC article.
-
Intravenous magnesium sulfate for vaso-occlusive episodes in sickle cell disease.Pediatrics. 2013 Dec;132(6):e1634-41. doi: 10.1542/peds.2013-2065. Epub 2013 Nov 25. Pediatrics. 2013. PMID: 24276838 Clinical Trial.
-
Magnesium for treating sickle cell disease.Cochrane Database Syst Rev. 2019 Sep 9;9(9):CD011358. doi: 10.1002/14651858.CD011358.pub3. Cochrane Database Syst Rev. 2019. PMID: 31498421 Free PMC article.
-
The effect of magnesium on length of stay for pediatric sickle cell pain crisis.Acad Emerg Med. 2004 Sep;11(9):968-72. doi: 10.1197/j.aem.2004.04.009. Acad Emerg Med. 2004. PMID: 15347549 Clinical Trial.
-
Ketamine infusion for sickle cell pain crisis refractory to opioids: a case report and review of literature.Ann Hematol. 2014 May;93(5):769-71. doi: 10.1007/s00277-013-1954-3. Epub 2013 Nov 15. Ann Hematol. 2014. PMID: 24232306 Review.
Cited by
-
Intranasal fentanyl and discharge from the emergency department among children with sickle cell disease and vaso-occlusive pain: A multicenter pediatric emergency medicine perspective.Am J Hematol. 2023 Apr;98(4):620-627. doi: 10.1002/ajh.26837. Epub 2023 Feb 6. Am J Hematol. 2023. PMID: 36606705 Free PMC article.
-
A randomized controlled trial comparing two vaso-occlusive episode (VOE) protocols in sickle cell disease (SCD).Am J Hematol. 2018 Feb;93(2):159-168. doi: 10.1002/ajh.24948. Epub 2017 Nov 10. Am J Hematol. 2018. PMID: 29047145 Free PMC article. Clinical Trial.
-
HABIT, a Randomized Feasibility Trial to Increase Hydroxyurea Adherence, Suggests Improved Health-Related Quality of Life in Youths with Sickle Cell Disease.J Pediatr. 2018 Jun;197:177-185.e2. doi: 10.1016/j.jpeds.2018.01.054. Epub 2018 Mar 20. J Pediatr. 2018. PMID: 29571930 Free PMC article. Clinical Trial.
-
Analgesic management of uncomplicated acute sickle-cell pain crisis in pediatrics: a systematic review and meta-analysis.J Pediatr (Rio J). 2020 Mar-Apr;96(2):142-158. doi: 10.1016/j.jped.2019.05.004. Epub 2019 Jul 24. J Pediatr (Rio J). 2020. PMID: 31351033 Free PMC article.
-
Molecular inflammatory expression profiles associated with the frequency of pain in individuals with sickle cell disease.Blood Adv. 2025 Aug 12;9(15):3790-3800. doi: 10.1182/bloodadvances.2024015085. Blood Adv. 2025. PMID: 40238896 Free PMC article.
References
-
- Brousseau DC, Panepinto JA, Nimmer M, Hoffmann RG. The number of people with sickle-cell disease in the United States: national and state estimates. Am J Hematol. 2010;85(1):77–78. - PubMed
-
- Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010;38(4 Suppl):S512–S521. - PubMed
-
- Panepinto JA, Brousseau DC, Hillery CA, Scott JP. Variation in hospitalizations and hospital length of stay in children with vaso-occlusive crises in sickle cell disease. Pediatr Blood Cancer. 2005;44(2):182–186. - PubMed
-
- Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325(1):11–16. - PubMed
-
- Brousseau DC, Owens PL, Mosso AL, Panepinto JA, Steiner CA. Acute care utilization and rehospitalizations for sickle cell disease. JAMA. 2010;303(13):1288–1294. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

