NR2B-dependent cyclophilin D translocation suppresses the recovery of synaptic transmission after oxygen-glucose deprivation
- PMID: 26232180
- PMCID: PMC4872600
- DOI: 10.1016/j.bbadis.2015.07.019
NR2B-dependent cyclophilin D translocation suppresses the recovery of synaptic transmission after oxygen-glucose deprivation
Abstract
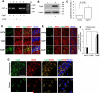
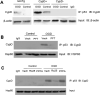
N-methyl d-aspartate receptor (NMDA) subunit 2B (NR2B)-containing NMDA receptors and mitochondrial protein cyclophilin D (CypD) are well characterized in mediating neuronal death after ischemia, respectively. However, whether and how NR2B and CypD work together in mediating synaptic injury after ischemia remains elusive. Using an ex vivo ischemia model of oxygen-glucose deprivation (OGD) in hippocampal slices, we identified a NR2B-dependent mechanism for CypD translocation onto the mitochondrial inner membrane. CypD depletion (CypD null mice) prevented OGD-induced impairment in synaptic transmission recovery. Overexpression of neuronal CypD mice (CypD+) exacerbated OGD-induced loss of synaptic transmission. Inhibition of CypD-dependent mitochondrial permeability transition pore (mPTP) opening by cyclosporine A (CSA) attenuated ischemia-induced synaptic perturbation in CypD+ and non-transgenic (non-Tg) mice. The treatment of antioxidant EUK134 to suppress mitochondrial oxidative stress rescued CypD-mediated synaptic dysfunction following OGD in CypD+ slices. Furthermore, OGD provoked the interaction of CypD with P53, which was enhanced in slices overexpressing CypD but was diminished in CypD-null slices. Inhibition of p53 using a specific inhibitor of p53 (pifithrin-μ) attenuated the CypD/p53 interaction following OGD, along with a restored synaptic transmission in both non-Tg and CypD+ hippocampal slices. Our results indicate that OGD-induced CypD translocation potentiates CypD/P53 interaction in a NR2B dependent manner, promoting oxidative stress and loss of synaptic transmission. We also evaluate a new ex vivo chronic OGD-induced ischemia model for studying the effect of oxidative stress on synaptic damage.
Keywords: CypD; Mitochondria; NR2B; OGD; Synaptic transmission; p53.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures





Similar articles
-
A Novel In Vitro CypD-Mediated p53 Aggregation Assay Suggests a Model for Mitochondrial Permeability Transition by Chaperone Systems.J Mol Biol. 2016 Oct 9;428(20):4154-4167. doi: 10.1016/j.jmb.2016.08.001. Epub 2016 Aug 8. J Mol Biol. 2016. PMID: 27515399 Free PMC article.
-
P53 dependent mitochondrial permeability transition pore opening is required for dexamethasone-induced death of osteoblasts.J Cell Physiol. 2014 Oct;229(10):1475-83. doi: 10.1002/jcp.24589. J Cell Physiol. 2014. PMID: 24615518
-
Oxygen glucose deprivation (OGD)/re-oxygenation-induced in vitro neuronal cell death involves mitochondrial cyclophilin-D/P53 signaling axis.Neurochem Res. 2013 Apr;38(4):705-13. doi: 10.1007/s11064-013-0968-5. Epub 2013 Jan 16. Neurochem Res. 2013. PMID: 23322110
-
Cyclophilin D: Guardian or Executioner for Tumor Cells?Front Oncol. 2022 Jul 4;12:939588. doi: 10.3389/fonc.2022.939588. eCollection 2022. Front Oncol. 2022. PMID: 35860554 Free PMC article. Review.
-
Potential enhancement of post-stroke angiogenic response by targeting the oligomeric aggregation of p53 protein.Front Cell Neurosci. 2023 Jul 18;17:1193362. doi: 10.3389/fncel.2023.1193362. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37534043 Free PMC article. Review.
Cited by
-
Disease Outcome and Brain Metabolomics of Cyclophilin-D Knockout Mice in Sepsis.Int J Mol Sci. 2022 Jan 16;23(2):961. doi: 10.3390/ijms23020961. Int J Mol Sci. 2022. PMID: 35055146 Free PMC article.
-
Mitochondrial Perturbation in Alzheimer's Disease and Diabetes.Prog Mol Biol Transl Sci. 2017;146:341-361. doi: 10.1016/bs.pmbts.2016.12.019. Epub 2017 Feb 4. Prog Mol Biol Transl Sci. 2017. PMID: 28253990 Free PMC article. Review.
-
Icaritin Alleviates Glutamate-Induced Neuronal Damage by Inactivating GluN2B-Containing NMDARs Through the ERK/DAPK1 Pathway.Front Neurosci. 2021 Feb 22;15:525615. doi: 10.3389/fnins.2021.525615. eCollection 2021. Front Neurosci. 2021. PMID: 33692666 Free PMC article.
-
Mitochondrial permeability transition pore: a potential drug target for neurodegeneration.Drug Discov Today. 2018 Dec;23(12):1983-1989. doi: 10.1016/j.drudis.2018.08.001. Epub 2018 Aug 3. Drug Discov Today. 2018. PMID: 30081095 Free PMC article. Review.
-
Mitochondrial Dysfunction Triggers Synaptic Deficits via Activation of p38 MAP Kinase Signaling in Differentiated Alzheimer's Disease Trans-Mitochondrial Cybrid Cells.J Alzheimers Dis. 2017;59(1):223-239. doi: 10.3233/JAD-170283. J Alzheimers Dis. 2017. PMID: 28598851 Free PMC article.
References
-
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. - PubMed
-
- Chan PH. Mitochondria and neuronal death/survival signaling pathways in cerebral ischemia. Neurochemical research. 2004;29:1943–1949. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

