Prefrontal microRNA-221 Mediates Environmental Enrichment-Induced Increase of Locomotor Sensitivity to Nicotine
- PMID: 26232787
- PMCID: PMC4772274
- DOI: 10.1093/ijnp/pyv090
Prefrontal microRNA-221 Mediates Environmental Enrichment-Induced Increase of Locomotor Sensitivity to Nicotine
Erratum in
-
Erratum.Int J Neuropsychopharmacol. 2016 Apr 27;19(10):pyw031. doi: 10.1093/ijnp/pyw031. Int J Neuropsychopharmacol. 2016. PMID: 27207904 Free PMC article. No abstract available.
Abstract
Background: Environmental enrichment alters susceptibility in developing drug addiction. We have demonstrated that rats raised in an enriched condition are more sensitive than rats raised in an impoverished condition to nicotine-induced locomotor activity, and this is associated with alterations of phosphorylated extracellular signal-regulated kinase 1/2 within the prefrontal cortex. This study determined the impact of microRNA-221 in the prefrontal cortex on phosphorylated extracellular signal-regulated kinase 1/2 and the enriched environment-dependent behavioral changes in response to nicotine.
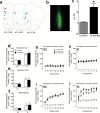
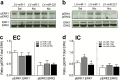
Methods: A microRNA array was conducted to profile microRNA expression in the prefrontal cortex of enriched condition and impoverished condition rats in response to repeated nicotine (0.35 mg/kg, s.c.) administration. microRNA-221 in the prefrontal cortex, nucleus accumbens, and striatum was further verified by quantitative real-time PCR. Lentiviral-mediated overexpression of microRNA-221 in PC12 cells and the medial prefrontal cortex was performed to determine the effects of microRNA-221 on nicotine-mediated phosphorylated extracellular signal-regulated kinase 1/2, phosphorylated cAMP-response element-binding protein, and locomotor activity.
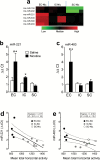
Results: microRNA-221 was profoundly upregulated in the prefrontal cortex but not in nucleus accumbens and striatum of enriched condition rats relative to impoverished condition rats following repeated administration of nicotine. Overexpression of lentiviral-microRNA-221 attenuated nicotine-induced increase in phosphorylated extracellular signal-regulated kinase 1/2 in PC12 cells. Lentiviral-microRNA-221 overexpression in the medial prefrontal cortex further increased locomotor activity in impoverished condition but not in enriched condition rats in response to repeated nicotine administration. Accordingly, lentiviral-microRNA-221 attenuated nicotine-induced increases in phosphorylated extracellular signal-regulated kinase 1/2 and phosphorylated cAMP-response element-binding protein in the medial prefrontal cortex of impoverished condition but not enriched condition rats.
Conclusion: These findings suggest that environmental enrichment, via upregulation of prefrontal microRNA-221 expression, suppresses the nicotine-induced activation of extracellular signal-regulated kinase and cAMP-response element-binding protein, which provides a potential mechanism underlying enhanced locomotor sensitivity to nicotine.
Keywords: ERK; Environmental enrichment; medial prefrontal cortex; microRNA; nicotine.
© The Author 2015. Published by Oxford University Press on behalf of CINP.
Figures



References
-
- Bahi A, Dreyer JL. (2013) Striatal modulation of BDNF expression using microRNA124a-expressing lentiviral vectors impairs ethanol-induced conditioned-place preference and voluntary alcohol consumption. Eur J Neurosci 38:2328–2337. - PubMed
-
- Bowling SL, Rowlett JK, Bardo MT. (1993) The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology 32:885–893. - PubMed
-
- Brondello JM, Pouyssegur J, McKenzie FR. (1999) Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science 286:2514–2517. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

