Impaired 2-AG Signaling in Hippocampal Glutamatergic Neurons: Aggravation of Anxiety-Like Behavior and Unaltered Seizure Susceptibility
- PMID: 26232789
- PMCID: PMC4772822
- DOI: 10.1093/ijnp/pyv091
Impaired 2-AG Signaling in Hippocampal Glutamatergic Neurons: Aggravation of Anxiety-Like Behavior and Unaltered Seizure Susceptibility
Erratum in
-
Erratum.Int J Neuropsychopharmacol. 2016 Apr 27;19(10):pyw031. doi: 10.1093/ijnp/pyw031. Int J Neuropsychopharmacol. 2016. PMID: 27207904 Free PMC article. No abstract available.
Abstract
Background: Postsynaptically generated 2-arachidonoylglycerol activates the presynaptic cannabinoid type-1 receptor, which is involved in synaptic plasticity at both glutamatergic and GABAergic synapses. However, the differential function of 2-arachidonoylglycerol signaling at glutamatergic vs GABAergic synapses in the context of animal behavior has not been investigated yet.
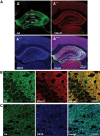
Methods: Here, we analyzed the role of 2-arachidonoylglycerol signaling selectively in hippocampal glutamatergic neurons. Monoacylglycerol lipase, the primary degrading enzyme of 2-arachidonoylglycerol, is expressed at presynaptic sites of excitatory and inhibitory neurons. By adeno-associated virus-mediated overexpression of monoacylglycerol lipase in glutamatergic neurons of the mouse hippocampus, we selectively interfered with 2-arachidonoylglycerol signaling at glutamatergic synapses of these neurons.
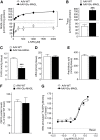
Results: Genetic modification of monoacylglycerol lipase resulted in a 50% decrease in 2-arachidonoylglycerol tissue levels without affecting the content of the second major endocannabinoid anandamide. A typical electrophysiological read-out for 2-arachidonoylglycerol signaling is the depolarization-induced suppression of excitation and of inhibition. Elevated monoacylglycerol lipase levels at glutamatergic terminals selectively impaired depolarization-induced suppression of excitation, while depolarization-induced suppression of inhibition was not significantly changed. At the behavioral level, mice with impaired hippocampal glutamatergic 2-arachidonoylglycerol signaling exhibited increased anxiety-like behavior but showed no alterations in aversive memory formation and seizure susceptibility.
Conclusion: Our data indicate that 2-arachidonoylglycerol signaling selectively in hippocampal glutamatergic neurons is essential for the animal's adaptation to aversive situations.
Keywords: Monoacylglycerol lipase; anxiety; endocannabinoids; epilepsy; hippocampus.
© The Author 2015. Published by Oxford University Press on behalf of CINP.
Figures




References
-
- Bagley J, Moghaddam B. (1997) Temporal dynamics of glutamate efflux in the prefrontal cortex and in the hippocampus following repeated stress: effects of pretreatment with saline or diazepam. Neurosci 77:65–73. - PubMed
-
- Coomber B, O’Donoghue MF, Mason R. (2008) Inhibition of endocannabinoid metabolism attenuates enhanced hippocampal neuronal activity induced by kainic acid. Synapse 62:746–755. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

