Aclidinium bromide and formoterol fumarate as a fixed-dose combination in COPD: pooled analysis of symptoms and exacerbations from two six-month, multicentre, randomised studies (ACLIFORM and AUGMENT)
- PMID: 26233481
- PMCID: PMC4531806
- DOI: 10.1186/s12931-015-0250-2
Aclidinium bromide and formoterol fumarate as a fixed-dose combination in COPD: pooled analysis of symptoms and exacerbations from two six-month, multicentre, randomised studies (ACLIFORM and AUGMENT)
Abstract
Background: The combination of aclidinium bromide, a long-acting anticholinergic, and formoterol fumarate, a long-acting beta2-agonist (400/12 μg twice daily) achieves improvements in lung function greater than either monotherapy in patients with chronic obstructive pulmonary disease (COPD), and is approved in the European Union as a maintenance treatment. The effect of this combination on symptoms of COPD and exacerbations is less well established. We examined these outcomes in a pre-specified analysis of pooled data from two 24-week, double-blind, parallel-group, active- and placebo-controlled, multicentre, randomised Phase III studies (ACLIFORM and AUGMENT).
Methods: Patients ≥40 years with moderate to severe COPD (post-bronchodilator forced expiratory volume in 1 s [FEV1]/forced vital capacity <70 % and FEV1 ≥30 % but <80 % predicted normal) were randomised (ACLIFORM: 2:2:2:2:1; AUGMENT: 1:1:1:1:1) to twice-daily aclidinium/formoterol 400/12 μg or 400/6 μg, aclidinium 400 μg, formoterol 12 μg or placebo via Genuair™/Pressair®. Dyspnoea (Transition Dyspnoea Index; TDI), daily symptoms (EXAcerbations of Chronic pulmonary disease Tool [EXACT]-Respiratory Symptoms [E-RS] questionnaire), night-time and early-morning symptoms, exacerbations (Healthcare Resource Utilisation [HCRU] and EXACT definitions) and relief-medication use were assessed.
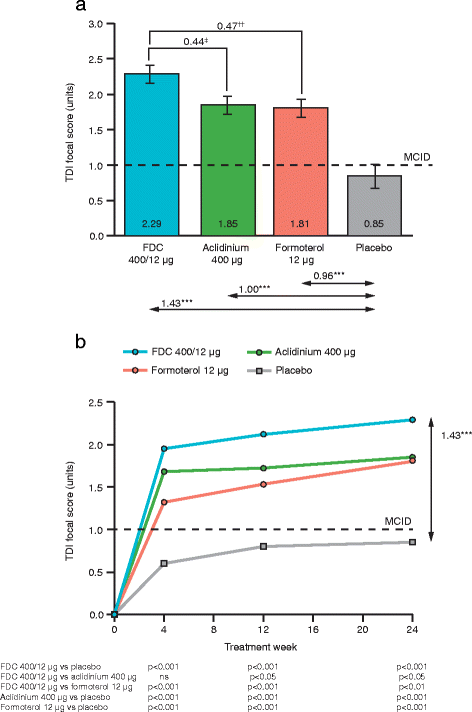
Results: The pooled intent-to-treat population included 3394 patients. Aclidinium/formoterol 400/12 μg significantly improved TDI focal score versus placebo and both monotherapies at Week 24 (all p < 0.05). Over 24 weeks, significant improvements in E-RS total score, overall night-time and early-morning symptom severity and limitation of early-morning activities were observed with aclidinium/formoterol 400/12 μg versus placebo and both monotherapies (all p < 0.05). The rate of moderate or severe HCRU exacerbations was significantly reduced with aclidinium/formoterol 400/12 μg compared with placebo (p < 0.05) but not monotherapies; the rate of EXACT-defined exacerbations was significantly reduced with aclidinium/formoterol 400/12 μg versus placebo (p < 0.01) and aclidinium (p < 0.05). Time to first HCRU or EXACT exacerbation was longer with aclidinium/formoterol 400/12 μg compared with placebo (all p < 0.05) but not the monotherapies. Relief-medication use was reduced with aclidinium/formoterol 400/12 μg versus placebo and aclidinium (p < 0.01).
Conclusions: Aclidinium/formoterol 400/12 μg significantly improves 24-hour symptom control compared with placebo, aclidinium and formoterol in patients with moderate to severe COPD. Furthermore, aclidinium/formoterol 400/12 μg reduces the frequency of exacerbations compared with placebo.
Trial registration: NCT01462942 and NCT01437397 (ClinicalTrials.gov).
Figures



References
-
- Global Initiative for Chronic Obstructive Lung Disease . Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. 2015. - PubMed
-
- Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins CR, Jones PW, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008;178:332–8. doi: 10.1164/rccm.200712-1869OC. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

