Antioxidant responses and cellular adjustments to oxidative stress
- PMID: 26233704
- PMCID: PMC4534574
- DOI: 10.1016/j.redox.2015.07.008
Antioxidant responses and cellular adjustments to oxidative stress
Abstract
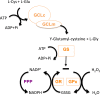
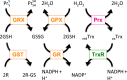
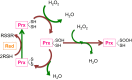
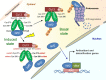
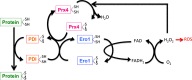
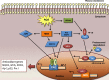
Redox biological reactions are now accepted to bear the Janus faceted feature of promoting both physiological signaling responses and pathophysiological cues. Endogenous antioxidant molecules participate in both scenarios. This review focuses on the role of crucial cellular nucleophiles, such as glutathione, and their capacity to interact with oxidants and to establish networks with other critical enzymes such as peroxiredoxins. We discuss the importance of the Nrf2-Keap1 pathway as an example of a transcriptional antioxidant response and we summarize transcriptional routes related to redox activation. As examples of pathophysiological cellular and tissular settings where antioxidant responses are major players we highlight endoplasmic reticulum stress and ischemia reperfusion. Topologically confined redox-mediated post-translational modifications of thiols are considered important molecular mechanisms mediating many antioxidant responses, whereas redox-sensitive microRNAs have emerged as key players in the posttranscriptional regulation of redox-mediated gene expression. Understanding such mechanisms may provide the basis for antioxidant-based therapeutic interventions in redox-related diseases.
Keywords: Antioxidants; ER stress; Ischemia–reperfusion; Redox signaling; Transcription factors.
Copyright © 2015. Published by Elsevier B.V.
Figures

References
-
- Hwang C., Sinskey A.J., Lodish H.F. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257(5076):1496–1502. - PubMed
-
- Pastore A., Piemonte F. S-glutathionylation signaling in cell biology: progress and prospects. Eur. J. Pharm. Sci. 2012;46(5):279–292. - PubMed
-
- Rahman I. Glutathione, stress responses, and redox signaling in lung inflammation. Antioxid. Redox Signal. 2005;7(1–2):42–59. - PubMed
-
- Haddad J.J., Harb H.L. L-gamma-glutamyl-l-cysteinyl-glycine (glutathione; GSH) and GSH-related enzymes in the regulation of pro- and anti-inflammatory cytokines: a signaling transcriptional scenario for redox(y) immunologic sensor(s)? Mol. Immunol. 2005;42(9):987–1014. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

