The PI3K/AKT Pathway and Renal Cell Carcinoma
- PMID: 26233890
- PMCID: PMC4624215
- DOI: 10.1016/j.jgg.2015.03.003
The PI3K/AKT Pathway and Renal Cell Carcinoma
Abstract
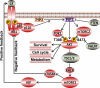
The phosphatidylinositol 3 kinase (PI3K)/AKT pathway is genetically targeted in more pathway components and in more tumor types than any other growth factor signaling pathway, and thus is frequently activated as a cancer driver. More importantly, the PI3K/AKT pathway is composed of multiple bifurcating and converging kinase cascades, providing many potential targets for cancer therapy. Renal cell carcinoma (RCC) is a high-risk and high-mortality cancer that is notoriously resistant to traditional chemotherapies or radiotherapies. The PI3K/AKT pathway is modestly mutated but highly activated in RCC, representing a promising drug target. Indeed, PI3K pathway inhibitors of the rapalog family are approved for use in RCC. Recent large-scale integrated analyses of a large number of patients have provided a molecular basis for RCC, reiterating the critical role of the PI3K/AKT pathway in this cancer. In this review, we summarize the genetic alterations of the PI3K/AKT pathway in RCC as indicated in the latest large-scale genome sequencing data, as well as treatments for RCC that target the aberrant activated PI3K/AKT pathway.
Keywords: AKT; PI3K; Renal cell carcinoma; Targeted therapy; mTOR.
Copyright © 2015 Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, and Genetics Society of China. Published by Elsevier Ltd. All rights reserved.
Figures
References
-
- Akbani R, Ng PKS, Werner HMJ, Shahmoradgoli M, Zhang F, Ju Z, Liu W, Yang J-Y, Yoshihara K, Li J, Ling S, Seviour EG, Ram PT, Minna JD, Diao L, Tong P, Heymach JV, Hill SM, Dondelinger F, Städler N, Byers LA, Meric-Bernstam F, Weinstein JN, Broom BM, Verhaak RGW, Liang H, Mukherjee S, Lu Y, Mills GB. A pan-cancer proteomic perspective on The Cancer Genome Atlas. Nat. Commun. 2014;5:3887. - PMC - PubMed
-
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B α. Curr. Biol. 1997;7:261–269. - PubMed
-
- Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 1997;272:31515–31524. - PubMed
-
- Auger KR, Serunian LA, Soltoff SP, Libby P, Cantley LC. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 1989;57:167–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

