RAG Represents a Widespread Threat to the Lymphocyte Genome
- PMID: 26234156
- PMCID: PMC4537821
- DOI: 10.1016/j.cell.2015.07.009
RAG Represents a Widespread Threat to the Lymphocyte Genome
Abstract
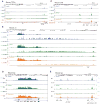
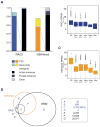
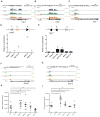
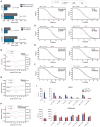
The RAG1 endonuclease, together with its cofactor RAG2, is essential for V(D)J recombination but is a potent threat to genome stability. The sources of RAG1 mis-targeting and the mechanisms that have evolved to suppress it are poorly understood. Here, we report that RAG1 associates with chromatin at thousands of active promoters and enhancers in the genome of developing lymphocytes. The mouse and human genomes appear to have responded by reducing the abundance of "cryptic" recombination signals near RAG1 binding sites. This depletion operates specifically on the RSS heptamer, whereas nonamers are enriched at RAG1 binding sites. Reversing this RAG-driven depletion of cleavage sites by insertion of strong recombination signals creates an ectopic hub of RAG-mediated V(D)J recombination and chromosomal translocations. Our findings delineate rules governing RAG binding in the genome, identify areas at risk of RAG-mediated damage, and highlight the evolutionary struggle to accommodate programmed DNA damage in developing lymphocytes.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures



References
-
- Biggin MD. Animal transcription networks as highly connected, quantitative continua. Dev Cell. 2011;21:611–626. - PubMed
-
- Bredemeyer AL, Sharma GG, Huang CY, Helmink BA, Walker LM, Khor KC, Nuskey B, Sullivan KE, Pandita TK, Bassing CH, et al. ATM stabilizes DNA double-strand-break complexes during V(D)J recombination. Nature. 2006;442:466–470. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

