Vertical stratification of matrix production is essential for physical integrity and architecture of macrocolony biofilms of Escherichia coli
- PMID: 26234179
- PMCID: PMC5014196
- DOI: 10.1111/1462-2920.12991
Vertical stratification of matrix production is essential for physical integrity and architecture of macrocolony biofilms of Escherichia coli
Abstract
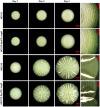
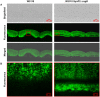
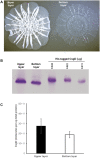
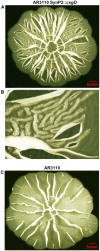
Bacterial macrocolony biofilms grow into intricate three-dimensional structures that depend on self-produced extracellular polymers conferring protection, cohesion and elasticity to the biofilm. In Escherichia coli, synthesis of this matrix - consisting of amyloid curli fibres and cellulose - requires CsgD, a transcription factor regulated by the stationary phase sigma factor RpoS, and occurs in the nutrient-deprived cells of the upper layer of macrocolonies. Is this asymmetric matrix distribution functionally important or is it just a fortuitous by-product of an unavoidable nutrient gradient? In order to address this question, the RpoS-dependent csgD promoter was replaced by a vegetative promoter. This re-wiring of csgD led to CsgD and matrix production in both strata of macrocolonies, with the lower layer transforming into a rigid 'base plate' of growing yet curli-connected cells. As a result, the two strata broke apart followed by desiccation and exfoliation of the top layer. By contrast, matrix-free cells at the bottom of wild-type macrocolonies maintain colony contact with the humid agar support by flexibly filling the space that opens up under buckling areas of the macrocolony. Precisely regulated stratification in matrix-free and matrix-producing cell layers is thus essential for the physical integrity and architecture of E. coli macrocolony biofilms.
© 2015 The Authors. Environmental Microbiology published by Society for Applied Microbiology and John Wiley & Sons Ltd.
Figures





References
-
- Allesen‐Holm, M. , Barken, K.B. , Yang, L. , Klausen, M. , Webb, J.S. , Kjelleberg, S. , et al (2006) A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol Microbiol 59: 1114–1128. - PubMed
-
- Branda, S.S. , Vik, A. , Friedman, L. , and Kolter, R. (2005) Biofilms: the matrix revisited. Trends Microbiol 13: 20–26. - PubMed
-
- Cerda, E. , and Mahadevan, L. (2003) Geometry and physics of wrinkling. Phys Rev Lett 90: 074302. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

