Restoration of Vision with Ectopic Expression of Human Rod Opsin
- PMID: 26234216
- PMCID: PMC4540256
- DOI: 10.1016/j.cub.2015.07.029
Restoration of Vision with Ectopic Expression of Human Rod Opsin
Abstract
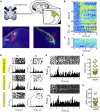
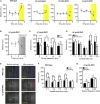
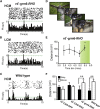
Many retinal dystrophies result in photoreceptor loss, but the inner retinal neurons can survive, making them potentially amenable to emerging optogenetic therapies. Here, we show that ectopically expressed human rod opsin, driven by either a non-selective or ON-bipolar cell-specific promoter, can function outside native photoreceptors and restore visual function in a mouse model of advanced retinal degeneration. Electrophysiological recordings from retinal explants and the visual thalamus revealed changes in firing (increases and decreases) induced by simple light pulses, luminance increases, and naturalistic movies in treated mice. These responses could be elicited at light intensities within the physiological range and substantially below those required by other optogenetic strategies. Mice with rod opsin expression driven by the ON-bipolar specific promoter displayed behavioral responses to increases in luminance, flicker, coarse spatial patterns, and elements of a natural movie at levels of contrast and illuminance (≈50-100 lux) typical of natural indoor environments. These data reveal that virally mediated ectopic expression of human rod opsin can restore vision under natural viewing conditions and at moderate light intensities. Given the inherent advantages in employing a human protein, the simplicity of this intervention, and the quality of vision restored, we suggest that rod opsin merits consideration as an optogenetic actuator for treating patients with advanced retinal degeneration.
Keywords: rhodopsin.
Copyright © 2015 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


Comment in
-
Vision Science: Can Rhodopsin Cure Blindness?Curr Biol. 2015 Aug 17;25(16):R713-5. doi: 10.1016/j.cub.2015.07.004. Curr Biol. 2015. PMID: 26294183
References
-
- Santos A., Humayun M.S., de Juan E., Jr., Greenburg R.J., Marsh M.J., Klock I.B., Milam A.H. Preservation of the inner retina in retinitis pigmentosa. A morphometric analysis. Arch. Ophthalmol. 1997;115:511–515. - PubMed
-
- Busskamp V., Picaud S., Sahel J.A., Roska B. Optogenetic therapy for retinitis pigmentosa. Gene Ther. 2012;19:169–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

