Transient sampling of aggregation-prone conformations causes pathogenic instability of a parkinsonian mutant of DJ-1 at physiological temperature
- PMID: 26234586
- PMCID: PMC4594666
- DOI: 10.1002/pro.2762
Transient sampling of aggregation-prone conformations causes pathogenic instability of a parkinsonian mutant of DJ-1 at physiological temperature
Abstract
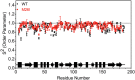
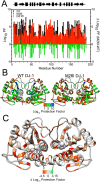
Various missense mutations in the cytoprotective protein DJ-1 cause rare forms of inherited parkinsonism. One mutation, M26I, diminishes DJ-1 protein levels in the cell but does not result in large changes in the three-dimensional structure or thermal stability of the protein. Therefore, the molecular defect that results in loss of M26I DJ-1 protective function is unclear. Using NMR spectroscopy near physiological temperature, we found that the picosecond-nanosecond dynamics of wild-type and M26I DJ-1 are similar. In contrast, elevated amide hydrogen/deuterium exchange rates indicate that M26I DJ-1 is more flexible than the wild-type protein on longer timescales and that hydrophobic regions of M26I DJ-1 are transiently exposed to solvent. Tryptophan fluorescence spectroscopy and thiol crosslinking analyzed by mass spectrometry also demonstrate that M26I DJ-1 samples conformations that differ from the wild-type protein at 37°C. These transiently sampled conformations are unstable and cause M26I DJ-1 to aggregate in vitro at physiological temperature but not at lower temperatures. M26I DJ-1 aggregation is correlated with pathogenicity, as the structurally similar but non-pathogenic M26L mutation does not aggregate at 37°C. The onset of dynamically driven M26I DJ-1 instability at physiological temperature resolves conflicting literature reports about the behavior of this disease-associated mutant and illustrates the pitfalls of characterizing proteins exclusively at room temperature or below, as key aspects of their behavior may not be apparent.
Keywords: DJ-1; PARK7; Parkinson's disease; conformational dynamics; protein stability; thiol crosslinking.
© 2015 The Protein Society.
Figures




References
-
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. - PubMed
-
- Greenamyre JT, Hastings TG. Biomedicine. Parkinson’s—divergent causes, convergent mechanisms. Science. 2004;304:1120–1122. - PubMed
-
- Jin J, Li GJ, Davis J, Zhu D, Wang Y, Pan C, Zhang J. Identification of novel proteins associated with both alpha-synuclein and DJ-1. Mol Cell Proteomics. 2007;6:845–859. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

