Taurine Boosts Cellular Uptake of Small D-Peptides for Enzyme-Instructed Intracellular Molecular Self-Assembly
- PMID: 26235707
- PMCID: PMC4544318
- DOI: 10.1021/jacs.5b06181
Taurine Boosts Cellular Uptake of Small D-Peptides for Enzyme-Instructed Intracellular Molecular Self-Assembly
Abstract
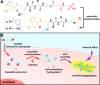
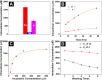
Due to their biostability, D-peptides are emerging as an important molecular platform for biomedical applications. Being proteolytically resistant, D-peptides lack interactions with endogenous transporters and hardly enter cells. Here we show that taurine, a natural amino acid, drastically boosts the cellular uptake of small D-peptides in mammalian cells by >10-fold, from 118 μM (without conjugating taurine) to >1.6 mM (after conjugating taurine). The uptake of a large amount of the ester conjugate of taurine and D-peptide allows intracellular esterase to trigger intracellular self-assembly of the D-peptide derivative, further enhancing their cellular accumulation. The study on the mechanism of the uptake reveals that the conjugates enter cells via both dynamin-dependent endocytosis and macropinocytosis, but likely not relying on taurine transporters. Differing fundamentally from the positively charged cell-penetrating peptides, the biocompatibility, stability, and simplicity of the enzyme-cleavable taurine motif promise new ways to promote the uptake of bioactive molecules for countering the action of efflux pump and contributing to intracellular molecular self-assembly.
Figures


References
-
- Reich Z.; Schramm O.; Brumfeld V.; Minsky A. J. Am. Chem. Soc. 1996, 118, 6345.10.1021/ja9600237. - DOI
- Morii T.; Tanaka T.; Sato S.-i.; Hagihara M.; Aizawa Y.; Makino K. J. Am. Chem. Soc. 2002, 124, 180.10.1021/ja017078f. - DOI - PubMed
- Michaud M.; Jourdan E.; Villet A.; Ravel A.; Grosset C.; Peyrin E. J. Am. Chem. Soc. 2003, 125, 8672.10.1021/ja034483t. - DOI - PubMed
-
- Eckert D. M.; Malashkevich V. N.; Hong L. H.; Carr P. A.; Kim P. S. Cell 1999, 99, 103.10.1016/S0092-8674(00)80066-5. - DOI - PubMed
- Schumacher T. N. M.; Mayr L. M.; Minor D. L.; Milhollen M. A.; Burgess M. W.; Kim P. S. Science 1996, 271, 1854.10.1126/science.271.5257.1854. - DOI - PubMed
- Fitzgerald M. C.; Chernushevich I.; Standing K. G.; Kent S. B. H.; Whitman C. P. J. Am. Chem. Soc. 1995, 117, 11075.10.1021/ja00150a006. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

