Blood-sampling collection prior to surgery may have a significant influence upon biomarker concentrations measured
- PMID: 26236175
- PMCID: PMC4521486
- DOI: 10.1186/s12014-015-9093-6
Blood-sampling collection prior to surgery may have a significant influence upon biomarker concentrations measured
Abstract
Background: Biomarkers can be subtle tools to aid the diagnosis, prognosis and monitoring of therapy and disease progression. The validation of biomarkers is a cumbersome process involving many steps. Serum samples from lung cancer patients were collected in the framework of a larger study for evaluation of biomarkers for early detection of lung cancer. The analysis of biomarker levels measured revealed a noticeable difference in certain biomarker values that exhibited a dependence of the time point and setting of the sampling. Biomarker concentrations differed significantly if taken before or after the induction of anesthesia and if sampled via venipuncture or arterial catheter.
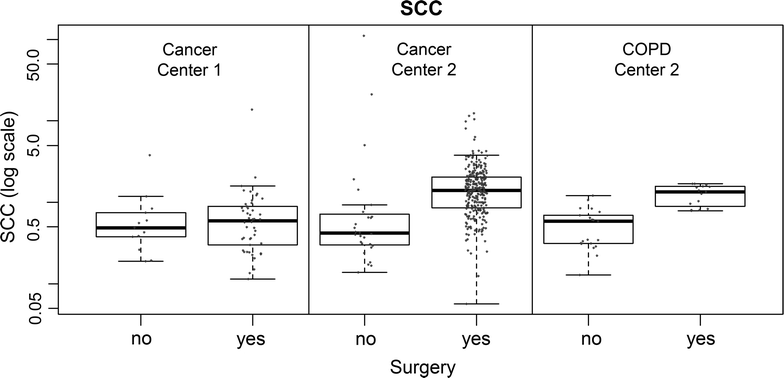
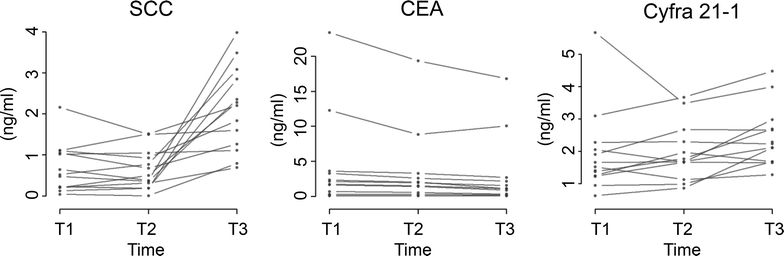
Methods: To investigate this observation, blood samples from 13 patients were drawn 1-2 days prior to surgery (T1), on the same day by venipuncture (T2) and after induction of anesthesia via arterial catheter (T3). The biomarkers Squamous Cell Carcinoma antigen (CanAG SCC EIA, Fujirebio Diagnostics, Malvern, USA), Carcinoembrionic Antigen (CEA), and CYFRA 21-1 (Roche Diagnostics GmbH, Mannheim, Germany) were analyzed.
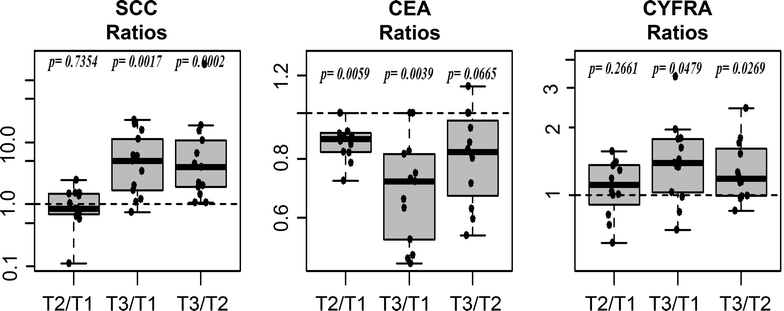
Results: SCC showed a very strong effect in relation to the sampling time and procedure. While the first two points in time (T1; T2) were highly comparable (median fold-change: 0.84; p = 0.7354; correlation ρ = 0.883), patients showed a significant increase (median fold-change: 4.96; p = 0.0017; correlation ρ = -0.036) in concentration when comparing T1 with the sample time subsequent to anesthesia induction (T3). A much weaker increase was found for CYFRA 21-1 at T3 (median fold-change: 1.40; p = 0.0479). The concentration of CEA showed a very small, but systematic decrease (median fold-change: 0.72; p = 0.0039).
Conclusions: In this study we show the unexpectedly marked influence of blood withdrawal timing (before vs. after anesthesia) and procedure (venous versus arterial vessel puncture) has on the concentration of the protein biomarker SCC and to a less extent upon CYFRA21-1. The potential causes for these effects remain to be elucidated in subsequent studies, however these findings highlight the importance of a standardized, controlled blood collection protocol for biomarker detection.
Keywords: Anesthesia induction; Biomarker; Blood specimens; CEA; CYFRA 21-1; Lung cancer; SCC; SPREC coding; Sampling time points.
Figures

Similar articles
-
The combination of the blood based tumor biomarkers cytokeratin 19 fragments (CYFRA 21-1) and carcinoembryonic antigen (CEA) as a potential predictor of benefit from adjuvant chemotherapy in early stage squamous cell carcinoma of the lung (SCC).Lung Cancer. 2018 Jun;120:46-53. doi: 10.1016/j.lungcan.2018.03.015. Epub 2018 Mar 17. Lung Cancer. 2018. PMID: 29748014
-
CEA, CYFRA21-1 and SCC in non-small cell lung cancer.Lung Cancer. 1995 Oct;13(2):169-76. doi: 10.1016/0169-5002(95)00485-8. Lung Cancer. 1995. PMID: 8581396
-
CYFRA 21-1 enzyme-linked immunosorbent assay. Evaluation as a tumor marker in non-small cell lung cancer.Chest. 1996 Apr;109(4):995-1000. doi: 10.1378/chest.109.4.995. Chest. 1996. PMID: 8635383
-
[The clinical signifinace of CEA, Cyfra21-1 and SCC in laryngeal carcinoma's clinicopathological parameters].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017 Aug 5;31(15):1182-1186. doi: 10.13201/j.issn.1001-1781.2017.15.010. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017. PMID: 29798354 Chinese.
-
Does the assessment of serum markers in patients with lung cancer aid in the clinical decision making process?Anticancer Res. 1996 Jul-Aug;16(4B):2161-8. Anticancer Res. 1996. PMID: 8694537 Review.
Cited by
-
Effects of ex vivo ischemia time and delayed processing on quality of specimens in tissue biobank.Mol Med Rep. 2020 Nov;22(5):4278-4288. doi: 10.3892/mmr.2020.11503. Epub 2020 Sep 10. Mol Med Rep. 2020. PMID: 33000275 Free PMC article.
-
Preoperative Fasting and General Anaesthesia Alter the Plasma Proteome.Cancers (Basel). 2020 Aug 27;12(9):2439. doi: 10.3390/cancers12092439. Cancers (Basel). 2020. PMID: 32867270 Free PMC article.
-
Serum markers improve current prediction of metastasis development in early-stage melanoma patients: a machine learning-based study.Mol Oncol. 2020 Aug;14(8):1705-1718. doi: 10.1002/1878-0261.12732. Epub 2020 Jun 24. Mol Oncol. 2020. PMID: 32485045 Free PMC article.
-
The Non-Cancer Specific Elevation of the Serum Squamous Cell Carcinoma Antigen during the Post-Radiotherapy Follow-Up of Cervical Cancer Patients.Diagnostics (Basel). 2021 Aug 31;11(9):1585. doi: 10.3390/diagnostics11091585. Diagnostics (Basel). 2021. PMID: 34573927 Free PMC article.
References
-
- Cabrera-Alarcon JL, Carrillo-Vico A, Santotoribio JD, Leon-Justel A, Sanchez-Gil R, Gonzalez-Castro A, et al. CYFRA 21-1 as a tool for distant metastasis detection in lung cancer. Clin Lab. 2011;57:1011–1014. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
