Interaction of electrically evoked activity with intrinsic dynamics of cultured cortical networks with and without functional fast GABAergic synaptic transmission
- PMID: 26236196
- PMCID: PMC4505148
- DOI: 10.3389/fncel.2015.00272
Interaction of electrically evoked activity with intrinsic dynamics of cultured cortical networks with and without functional fast GABAergic synaptic transmission
Abstract
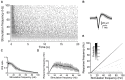
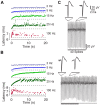
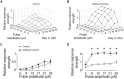
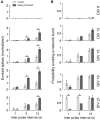
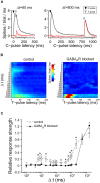
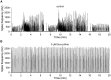
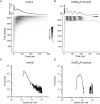
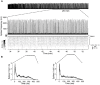
The modulation of neuronal activity by means of electrical stimulation is a successful therapeutic approach for patients suffering from a variety of central nervous system disorders. Prototypic networks formed by cultured cortical neurons represent an important model system to gain general insights in the input-output relationships of neuronal tissue. These networks undergo a multitude of developmental changes during their maturation, such as the excitatory-inhibitory shift of the neurotransmitter GABA. Very few studies have addressed how the output properties to a given stimulus change with ongoing development. Here, we investigate input-output relationships of cultured cortical networks by probing cultures with and without functional GABAAergic synaptic transmission with a set of stimulation paradigms at various stages of maturation. On the cellular level, low stimulation rates (<15 Hz) led to reliable neuronal responses; higher rates were increasingly ineffective. Similarly, on the network level, lowest stimulation rates (<0.1 Hz) lead to maximal output rates at all ages, indicating a network wide refractory period after each stimulus. In cultures aged 3 weeks and older, a gradual recovery of the network excitability within tens of milliseconds was in contrast to an abrupt recovery after about 5 s in cultures with absent GABAAergic synaptic transmission. In these GABA deficient cultures evoked responses were prolonged and had multiple discharges. Furthermore, the network excitability changed periodically, with a very slow spontaneous change of the overall network activity in the minute range, which was not observed in cultures with absent GABAAergic synaptic transmission. The electrically evoked activity of cultured cortical networks, therefore, is governed by at least two potentially interacting mechanisms: A refractory period in the order of a few seconds and a very slow GABA dependent oscillation of the network excitability.
Keywords: MEA; cell culture; cerebral cortex; gamma-aminobutyric acid; multielectrode arrays; neocortex; network activity; stimulation.
Figures

Similar articles
-
Contribution of GABAergic interneurons to the development of spontaneous activity patterns in cultured neocortical networks.Front Cell Neurosci. 2010 Jun 21;4:15. doi: 10.3389/fncel.2010.00015. eCollection 2010. Front Cell Neurosci. 2010. PMID: 20617185 Free PMC article.
-
Synaptic activation of GABA(B) receptors regulates neuronal network activity and entrainment.Eur J Neurosci. 2007 May;25(10):2982-90. doi: 10.1111/j.1460-9568.2007.05544.x. Eur J Neurosci. 2007. PMID: 17561812
-
Quantitative examination of stimulus-response relations in cortical networks in vitro.J Neurophysiol. 2013 Apr;109(7):1764-74. doi: 10.1152/jn.00481.2012. Epub 2012 Dec 28. J Neurophysiol. 2013. PMID: 23274313
-
Compensatory physiological responses to chronic blockade of amino acid receptors during early development in spontaneously active organotypic cerebral cortex explants cultured in vitro.Prog Brain Res. 2005;147:231-48. doi: 10.1016/S0079-6123(04)47018-6. Prog Brain Res. 2005. PMID: 15581710 Review.
-
Physiological consequences of selective suppression of synaptic transmission in developing cerebral cortical networks in vitro: differential effects on intrinsically generated bioelectric discharges in a living 'model' system for slow-wave sleep activity.Neurosci Biobehav Rev. 2008 Oct;32(8):1569-600. doi: 10.1016/j.neubiorev.2008.06.008. Epub 2008 Jul 5. Neurosci Biobehav Rev. 2008. PMID: 18722467 Review.
Cited by
-
The Structural E/I Balance Constrains the Early Development of Cortical Network Activity.Front Cell Neurosci. 2021 Jul 19;15:687306. doi: 10.3389/fncel.2021.687306. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34349623 Free PMC article.
-
Inhibition increases response variability and reduces stimulus discrimination in random networks of cortical neurons.Sci Rep. 2019 Mar 21;9(1):4969. doi: 10.1038/s41598-019-41220-2. Sci Rep. 2019. PMID: 30899035 Free PMC article.
-
Myasthenia Gravis-An Analysis of Multimodal Evoked Potentials.Brain Sci. 2021 Aug 11;11(8):1057. doi: 10.3390/brainsci11081057. Brain Sci. 2021. PMID: 34439676 Free PMC article.
-
Unique Features of Network Bursts Emerge From the Complex Interplay of Excitatory and Inhibitory Receptors in Rat Neocortical Networks.Front Cell Neurosci. 2019 Sep 6;13:377. doi: 10.3389/fncel.2019.00377. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31555093 Free PMC article.
References
-
- Arnold F. J., Hofmann F., Bengtson C. P., Wittmann M., Vanhoutte P., Bading H. (2005). Microelectrode array recordings of cultured hippocampal networks reveal a simple model for transcription and protein synthesis-dependent plasticity. J. Physiol. 564 3–19. 10.1113/jphysiol.2004.077446 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

