An excitatory GABA loop operating in vivo
- PMID: 26236197
- PMCID: PMC4503922
- DOI: 10.3389/fncel.2015.00275
An excitatory GABA loop operating in vivo
Abstract
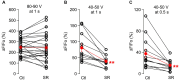
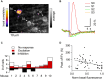
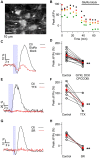
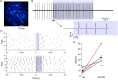
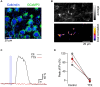
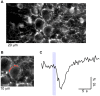
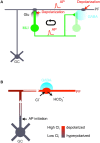
While it has been proposed that the conventional inhibitory neurotransmitter GABA can be excitatory in the mammalian brain, much remains to be learned concerning the circumstances and the cellular mechanisms governing potential excitatory GABA action. Using a combination of optogenetics and two-photon calcium imaging in vivo, we find that activation of chloride-permeable GABAA receptors in parallel fibers (PFs) of the cerebellar molecular layer of adult mice causes parallel fiber excitation. Stimulation of PFs at submaximal stimulus intensities leads to GABA release from molecular layer interneurons (MLIs), thus creating a positive feedback loop that enhances excitation near the center of an activated PF bundle. Our results imply that elevated chloride concentration can occur in specific intracellular compartments of mature mammalian neurons and suggest an excitatory role for GABAA receptors in the cerebellar cortex of adult mice.
Keywords: GABA; calcium; cerebellum; interneurons; parallel fibers.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

