Redox regulation of cGMP-dependent protein kinase Iα in the cardiovascular system
- PMID: 26236235
- PMCID: PMC4505079
- DOI: 10.3389/fphar.2015.00139
Redox regulation of cGMP-dependent protein kinase Iα in the cardiovascular system
Abstract
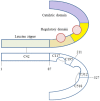
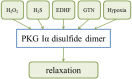
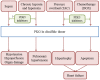
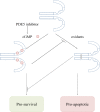
Elevated levels of oxidants in biological systems have been historically referred to as "oxidative stress," a choice of words that perhaps conveys an imbalanced view of reactive oxygen species in cells and tissues. The term stress suggests a harmful role, whereas a contemporary view is that oxidants are also crucial for the maintenance of homeostasis or adaptive signaling that can actually limit injury. This regulatory role for oxidants is achieved in part by them inducing oxidative post-translational modifications of proteins which may alter their function or interactions. Such mechanisms allow changes in cell oxidant levels to be coupled to regulated alterations in enzymatic function (i.e., signal transduction), which enables "redox signaling." In this review we focus on the role of cGMP-dependent protein kinase (PKG) Ia disulfide dimerisation, an oxidative modification that is induced by oxidants that directly activates the enzyme, discussing how this impacts on the cardiovascular system. Additionally, how this oxidative activation of PKG may coordinate with or differ from classical activation of this kinase by cGMP is also considered.
Keywords: cGMP; cardio-vascular system; disulfide dimerisation; oxidative modification; protein kinase G.
Figures
Similar articles
-
Examining a role for PKG Iα oxidation in the pathogenesis of cardiovascular dysfunction during diet-induced obesity.Free Radic Biol Med. 2017 Sep;110:390-398. doi: 10.1016/j.freeradbiomed.2017.07.007. Epub 2017 Jul 6. Free Radic Biol Med. 2017. PMID: 28690194 Free PMC article.
-
Cysteine redox sensor in PKGIa enables oxidant-induced activation.Science. 2007 Sep 7;317(5843):1393-7. doi: 10.1126/science.1144318. Epub 2007 Aug 23. Science. 2007. PMID: 17717153
-
Oxidation of cysteine 117 stimulates constitutive activation of the type Iα cGMP-dependent protein kinase.J Biol Chem. 2018 Oct 26;293(43):16791-16802. doi: 10.1074/jbc.RA118.004363. Epub 2018 Sep 11. J Biol Chem. 2018. PMID: 30206122 Free PMC article.
-
Hydrogen peroxide sensing and signaling by protein kinases in the cardiovascular system.Antioxid Redox Signal. 2013 Mar 20;18(9):1042-52. doi: 10.1089/ars.2012.4817. Epub 2012 Sep 17. Antioxid Redox Signal. 2013. PMID: 22867279 Free PMC article. Review.
-
Cyclic GMP and protein kinase-G in myocardial ischaemia-reperfusion: opportunities and obstacles for survival signaling.Br J Pharmacol. 2007 Nov;152(6):855-69. doi: 10.1038/sj.bjp.0707409. Epub 2007 Aug 13. Br J Pharmacol. 2007. PMID: 17700722 Free PMC article. Review.
Cited by
-
Endothelial-to-Mesenchymal Transition in Pulmonary Arterial Hypertension.Antioxid Redox Signal. 2021 Apr 20;34(12):891-914. doi: 10.1089/ars.2020.8169. Antioxid Redox Signal. 2021. PMID: 32746619 Free PMC article. Review.
-
Proof of Principle for a Novel Class of Antihypertensives That Target the Oxidative Activation of PKG Iα (Protein Kinase G Iα).Hypertension. 2017 Sep;70(3):577-586. doi: 10.1161/HYPERTENSIONAHA.117.09670. Epub 2017 Jul 17. Hypertension. 2017. PMID: 28716990 Free PMC article.
-
New Therapeutics for Heart Failure: Focusing on cGMP Signaling.Int J Mol Sci. 2023 Aug 16;24(16):12866. doi: 10.3390/ijms241612866. Int J Mol Sci. 2023. PMID: 37629047 Free PMC article. Review.
-
Oxidant sensor in the cGMP-binding pocket of PKGIα regulates nitroxyl-mediated kinase activity.Sci Rep. 2017 Aug 30;7(1):9938. doi: 10.1038/s41598-017-09275-1. Sci Rep. 2017. PMID: 28855531 Free PMC article.
-
The Emerging Roles of Nicotinamide Adenine Dinucleotide Phosphate Oxidase 2 in Skeletal Muscle Redox Signaling and Metabolism.Antioxid Redox Signal. 2019 Dec 20;31(18):1371-1410. doi: 10.1089/ars.2018.7678. Epub 2019 Nov 1. Antioxid Redox Signal. 2019. PMID: 31588777 Free PMC article. Review.
References
-
- Blanton R. M., Takimoto E., Lane A. M., Aronovitz M., Piotrowski R., Karas R. H., et al. (2012). Protein kinase g Iα inhibits pressure overload-induced cardiac remodeling and is required for the cardioprotective effect of sildenafil in vivo. J. Am. Heart. Assoc. 1, e003731. 10.1161/JAHA.112.003731 - DOI - PMC - PubMed
Publication types
Grants and funding
- RG/12/12/29872/BHF_/British Heart Foundation/United Kingdom
- G0700320/MRC_/Medical Research Council/United Kingdom
- MR/K003232/1/MRC_/Medical Research Council/United Kingdom
- BB/C503646/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- PG/15/26/31373/BHF_/British Heart Foundation/United Kingdom
- G0600785/MRC_/Medical Research Council/United Kingdom
- G1000458/MRC_/Medical Research Council/United Kingdom
- FS/11/45/28859/BHF_/British Heart Foundation/United Kingdom
- PG/13/13/30018/BHF_/British Heart Foundation/United Kingdom
- MR/L009684/1/MRC_/Medical Research Council/United Kingdom
- PG/10/98/28655/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

