Alleviation of carbon catabolite repression in Enterobacter aerogenes for efficient utilization of sugarcane molasses for 2,3-butanediol production
- PMID: 26236395
- PMCID: PMC4521459
- DOI: 10.1186/s13068-015-0290-3
Alleviation of carbon catabolite repression in Enterobacter aerogenes for efficient utilization of sugarcane molasses for 2,3-butanediol production
Abstract
Background: Due to its cost-effectiveness and rich sugar composition, sugarcane molasses is considered to be a promising carbon source for biorefinery. However, the sugar mixture in sugarcane molasses is not consumed as efficiently as glucose in microbial fermentation due to complex interactions among their utilizing pathways, such as carbon catabolite repression (CCR). In this study, 2,3-butanediol-producing Enterobacter aerogenes was engineered to alleviate CCR and improve sugar utilization by modulating its carbon preference.
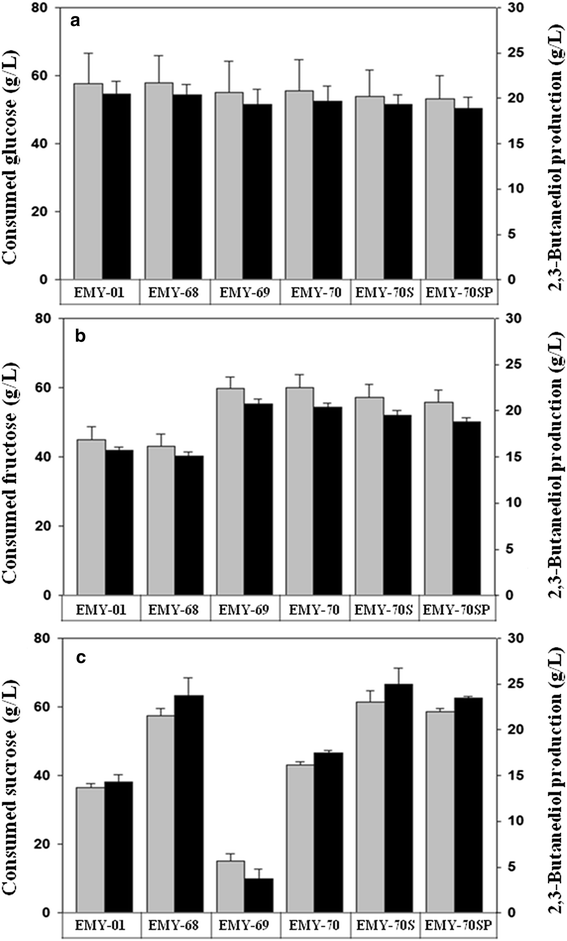
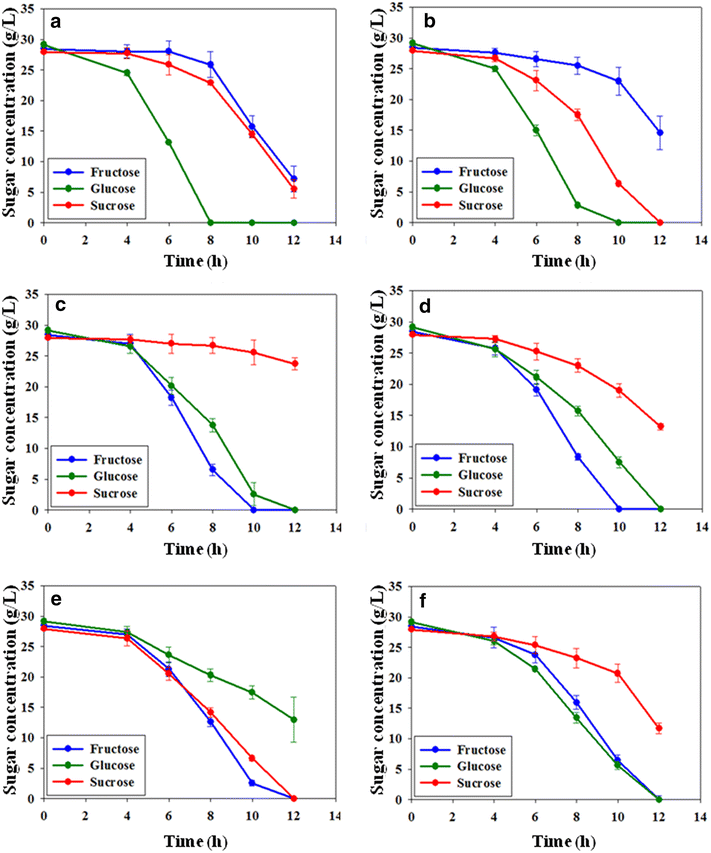
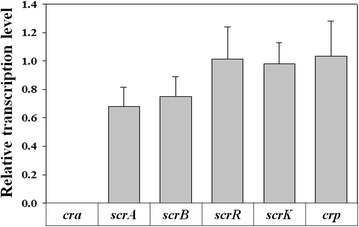
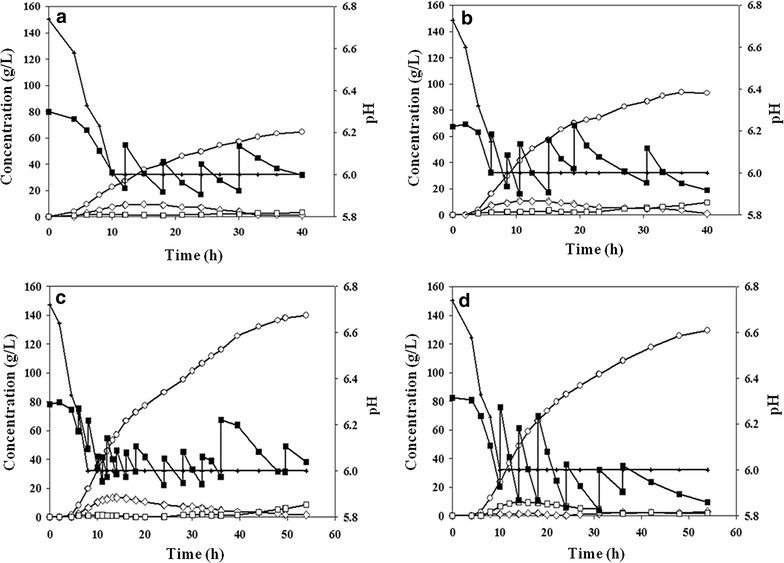
Results: The gene encoding catabolite repressor/activator (Cra) was deleted in the genome of E. aerogenes to increase the fructose consumption rate. However, the deletion mutation repressed sucrose utilization, resulting in the accumulation of sucrose in the fermentation medium. Cra regulation on expression of the scrAB operon involved in sucrose catabolism was verified by reverse transcription and real-time PCR, and the efficiency of sucrose utilization was restored by disrupting the scrR gene and overexpressing the scrAB operon. In addition, overexpression of the ptsG gene involved in glucose utilization enhanced the glucose preference among mixed sugars, which relieved glucose accumulation in fed-batch fermentation. In fed-batch fermentation using sugarcane molasses, the maximum titer of 2,3-butanediol production by the mutant reached 140.0 g/L at 54 h, which was by far the highest titer of 2,3-butanediol with E. aerogenes achieved through genetic engineering.
Conclusions: We have developed genetically engineered E. aerogenes as a 2,3-butanediol producer that efficiently utilizes sugarcane molasses. The fermentation efficiency was dramatically improved by the alleviation of CCR and modulation of carbon preference. These results offer a metabolic engineering approach for achieving highly efficient utilization of mixed sugars for the biorefinery industry.
Keywords: 2,3-Butanediol; Carbon catabolite repression; Catabolite repressor/activator; Enterobacter aerogenes; Fed-batch fermentation; Sugarcane molasses.
Figures


References
-
- van Haveren J, Scott EL, Sanders J. Bulk chemicals from biomass. Biofuel Bioprod Biorefin. 2008;2:41–57. doi: 10.1002/bbb.43. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

