Uterine Intravenous Leiomyomatosis with Cardiac Extension: Radiologic Assessment with Surgical and Pathologic Correlation
- PMID: 26236515
- PMCID: PMC4506837
- DOI: 10.1155/2015/576743
Uterine Intravenous Leiomyomatosis with Cardiac Extension: Radiologic Assessment with Surgical and Pathologic Correlation
Abstract
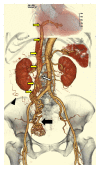
We present the computed tomography (CT) and magnetic resonance (MR) imaging findings of a 71-year-old woman with a cardiac extension of intravenous leiomyoma (IVL) that arose from the uterus, extended to the inferior vena cava (IVC), and reached the right ventricle through the right ovarian vein. Radiologic-pathologic correlation showed that the intravascular cord-like mass originating from the IVC and extending to the right ventricle was composed of degenerated smooth muscle cells with a number of large vessels that were regarded as arteries; moreover, the arteries within the cord-like mass appeared to be looping internally. Given the disappearance of the right ovarian venous wall around the IVL pathologically, extracting the tumor from the ovarian vein during an operation is considered to be impossible retrospectively. Also it was difficult to identify even the intravenous extension of the uterine leiomyoma histopathologically. Therefore, contrast-enhanced CT, in particular arterial phase imaging, provided important information that revealed the mass, range, and path of the lesion, ensuring that an appropriate operative plan could be drawn up and the tumor completely excised.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources

