Intrahepatic Cholestasis of Pregnancy and Serum Bile Acids in HIV-Infected Pregnant Women
- PMID: 26236558
- PMCID: PMC4519986
- DOI: 10.4172/2155-6113.1000464
Intrahepatic Cholestasis of Pregnancy and Serum Bile Acids in HIV-Infected Pregnant Women
Abstract
Objectives: Intra-hepatic cholestasis of pregnancy (ICP) is uncommon, but has severe effects on pregnancy outcomes. ICP is characterized by elevated serum bile acids and liver enzymes and preferentially affects women with liver disorders. We compared bile acids and pregnancy outcomes of HIV-infected pregnant women, who commonly have elevated live enzymes, with uninfected controls.
Methods: Twenty-four HIV-infected, including 2 co-infected with hepatitis C virus (HCV), and 25 uninfected women were tested during early and late pregnancy and postpartum.
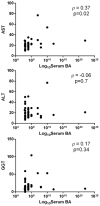
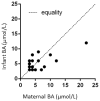
Results: After exclusion of the HCV-infected women, serum bile acids were similar in HIV-infected and uninfected participants. -glutamyl transpeptidase was elevated in HIV-infected compared with uninfected women during pregnancy and postpartum. Bilirubin and aspartate transaminase were higher in uninfected compared with HIV-infected women in early pregnancy, but subsequently similar. Bile acids in late pregnancy correlated with bile acids in the baby at birth. An HIV- and HCV-co-infected pregnant woman with active hepatitis developed ICP complicated by fetal distress. Another co-infected participant without active hepatitis had an uneventful pregnancy and delivery.
Conclusion: In the absence of HCV co-infection, bile acid metabolism appeared to be similar in HIV-infected and uninfected pregnant women. Both HIV-infected and uninfected pregnant women had mild liver enzyme elevations.
Keywords: Antiretroviral Therapy; Bile acids; Hepatitis C virus; Human Immunodeficiency Virus; Intrahepatic cholestasis; Liver function; Pregnancy.
Conflict of interest statement
The authors do not have any conflicts of interest.
Figures
Similar articles
-
The concentrations of bile acids and erythropoietin in pregnant women with intrahepatic cholestasis and the state of the fetus and newborn.Med Wieku Rozwoj. 2013 Jul-Sep;17(3):232-45. Med Wieku Rozwoj. 2013. PMID: 24296447
-
Intrahepatic cholestasis of pregnancy with concomitant hepatitis C virus infection, Joan C. Edwards SOM, Marshall University.Eur J Gastroenterol Hepatol. 2015 Apr;27(4):372-4. doi: 10.1097/MEG.0000000000000293. Eur J Gastroenterol Hepatol. 2015. PMID: 25874507
-
[Clinical practice guidelines of the Team of Experts of the Polish Gynecological Society: management of the intrahepatic cholestasis of pregnancy].Ginekol Pol. 2012 Sep;83(9):713-7. Ginekol Pol. 2012. PMID: 23342903 Polish.
-
Intrahepatic cholestasis of pregnancy (ICP): case report and review of the literature.Z Gastroenterol. 2016 Dec;54(12):1327-1333. doi: 10.1055/s-0042-118388. Epub 2016 Dec 9. Z Gastroenterol. 2016. PMID: 27936482 Review. English.
-
Bile Acids in Intrahepatic Cholestasis of Pregnancy.Diagnostics (Basel). 2022 Nov 9;12(11):2746. doi: 10.3390/diagnostics12112746. Diagnostics (Basel). 2022. PMID: 36359589 Free PMC article. Review.
References
-
- Pathak B, Sheibani L, Lee RH. Cholestasis of pregnancy. Obstet Gynecol Clin North Am. 2010;37:269–282. - PubMed
-
- Leslie KK, Reznikov L, Simon FR, Fennessey PV, Reyes H, et al. Estrogens in intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2000;95:372–376. - PubMed
-
- Reyes H, Sjövall J. Bile acids and progesterone metabolites in intrahepatic cholestasis of pregnancy. Ann Med. 2000;32:94–106. - PubMed
-
- Carter J. Serum bile acids in normal pregnancy. Br J Obstet Gynaecol. 1991;98:540–543. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
