A dimensionless ordered pull-through model of the mammalian lens epithelium evidences scaling across species and explains the age-dependent changes in cell density in the human lens
- PMID: 26236824
- PMCID: PMC4528606
- DOI: 10.1098/rsif.2015.0391
A dimensionless ordered pull-through model of the mammalian lens epithelium evidences scaling across species and explains the age-dependent changes in cell density in the human lens
Abstract
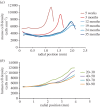
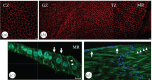
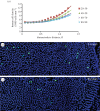
We present a mathematical (ordered pull-through; OPT) model of the cell-density profile for the mammalian lens epithelium together with new experimental data. The model is based upon dimensionless parameters, an important criterion for inter-species comparisons where lens sizes can vary greatly (e.g. bovine (approx. 18 mm); mouse (approx. 2 mm)) and confirms that mammalian lenses scale with size. The validated model includes two parameters: β/α, which is the ratio of the proliferation rate in the peripheral and in the central region of the lens; and γ(GZ), a dimensionless pull-through parameter that accounts for the cell transition and exit from the epithelium into the lens body. Best-fit values were determined for mouse, rat, rabbit, bovine and human lens epithelia. The OPT model accounts for the peak in cell density at the periphery of the lens epithelium, a region where cell proliferation is concentrated and reaches a maximum coincident with the germinative zone. The β/α ratio correlates with the measured FGF-2 gradient, a morphogen critical to lens cell survival, proliferation and differentiation. As proliferation declines with age, the OPT model predicted age-dependent changes in cell-density profiles, which we observed in mouse and human lenses.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

