Metabolic and evolutionary origin of actin-binding polyketides from diverse organisms
- PMID: 26236936
- PMCID: PMC7116039
- DOI: 10.1038/nchembio.1870
Metabolic and evolutionary origin of actin-binding polyketides from diverse organisms
Abstract
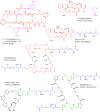
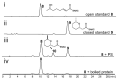
Actin-targeting macrolides comprise a large, structurally diverse group of cytotoxins isolated from remarkably dissimilar micro- and macroorganisms. In spite of their disparate origins and structures, many of these compounds bind actin at the same site and exhibit structural relationships reminiscent of modular, combinatorial drug libraries. Here we investigate biosynthesis and evolution of three compound groups: misakinolides, scytophycin-type compounds and luminaolides. For misakinolides from the sponge Theonella swinhoei WA, our data suggest production by an uncultivated 'Entotheonella' symbiont, further supporting the relevance of these bacteria as sources of bioactive polyketides and peptides in sponges. Insights into misakinolide biosynthesis permitted targeted genome mining for other members, providing a cyanobacterial luminaolide producer as the first cultivated source for this dimeric compound family. The data indicate that this polyketide family is bacteria-derived and that the unusual macrolide diversity is the result of combinatorial pathway modularity for some compounds and of convergent evolution for others.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Sasse F, Steinmetz H, Höfle G, Reichenbach H. Rhizopodin, a new compound from Myxococcus stipitatus (myxobacteria) causes formation of rhizopodia-like structures in animal cell cultures. Production, isolation, physico-chemical and biological properties. J Antibiot. 1993;46:741–748. - PubMed
-
- Ishibashi M, Moore RE, Patterson GML, Xu CF, Clardy J. Scytophycins, cytotoxic and antimycotic agents from the cyanophyte Scytonema pseudohofmanni . J Org Chem. 1986;51:5300–5306.
-
- Andrianasolo EH, et al. Isolation of swinholide A and related glycosylated derivatives from two field collections of marine cyanobacteria. Org Lett. 2005;7:1375–1378. - PubMed
-
- Sakai R, Higa T, Kashman Y. Misakinolide A, an antitumor macrolide from the marine sponge Theonella sp. Chem Lett. 1986:1499–1502.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- PubChem-Substance/252106873
LinkOut - more resources
Full Text Sources
Other Literature Sources

