Isolation of Mouse Neutrophils
- PMID: 26237011
- PMCID: PMC4574512
- DOI: 10.1002/0471142735.im0320s110
Isolation of Mouse Neutrophils
Abstract
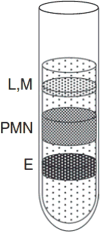
Neutrophils represent the first line of defense against bacterial and fungal pathogens. Indeed, patients with inherited and acquired qualitative and quantitative neutrophil defects are at high risk for developing bacterial and fungal infections and suffering adverse outcomes from these infections. Therefore, research aiming at defining the molecular factors that modulate neutrophil effector function under homeostatic conditions and during infection is essential for devising strategies to augment neutrophil function and improve the outcome of infected individuals. This unit describes a reproducible density gradient centrifugation-based protocol that can be applied in any laboratory to harvest large numbers of highly enriched and highly viable neutrophils from the bone marrow of mice both at the steady state and following infection with Candida albicans as described in UNIT. In another protocol, we also present a method that combines gentle enzymatic tissue digestion with a positive immunomagnetic selection technique or Fluorescence-activated cell sorting (FACS) to harvest highly pure and highly viable preparations of neutrophils directly from mouse tissues such as the kidney, the liver or the spleen. Finally, methods for isolating neutrophils from mouse peritoneal fluid and peripheral blood are included. Mouse neutrophils isolated by these protocols can be used for examining several aspects of cellular function ex vivo including pathogen binding, phagocytosis and killing, neutrophil chemotaxis, oxidative burst, degranulation and cytokine production, and for performing neutrophil adoptive transfer experiments.
Keywords: density gradient centrifugation; isolation; magnetic separation; mouse bone marrow; mouse tissue; neutrophils; sorting.
Copyright © 2015 John Wiley & Sons, Inc.
Figures


References
-
- Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annual review of immunology. 2012;30:459–489. - PubMed
-
- Boxio R, Bossenmeyer-Pourie C, Steinckwich N, Dournon C, Nusse O. Mouse bone marrow contains large numbers of functionally competent neutrophils. Journal of leukocyte biology. 2004;75:604–611. - PubMed
-
- Clark RA, Nauseef WM. Isolation and functional analysis of neutrophils. Current protocols in immunology / edited by John E Coligan … [et al] 2001:23. Chapter 7: Unit 7. - PubMed
-
- Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. Journal of leukocyte biology. 2008;83:64–70. - PubMed
-
- Devi S, Laning J, Luo Y, Dorf ME. Biologic activities of the β-chemokine TCA3 on neutrophils and macrophages. J Immunol. 1995;154:5376–5383. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

