Pediatric AML: From Biology to Clinical Management
- PMID: 26237023
- PMCID: PMC4470244
- DOI: 10.3390/jcm4010127
Pediatric AML: From Biology to Clinical Management
Abstract
Pediatric acute myeloid leukemia (AML) represents 15%-20% of all pediatric acute leukemias. Survival rates have increased over the past few decades to ~70%, due to improved supportive care, optimized risk stratification and intensified chemotherapy. In most children, AML presents as a de novo entity, but in a minority, it is a secondary malignancy. The diagnostic classification of pediatric AML includes a combination of morphology, cytochemistry, immunophenotyping and molecular genetics. Outcome is mainly dependent on the initial response to treatment and molecular and cytogenetic aberrations. Treatment consists of a combination of intensive anthracycline- and cytarabine-containing chemotherapy and stem cell transplantation in selected genetic high-risk cases or slow responders. In general, ~30% of all pediatric AML patients will suffer from relapse, whereas 5%-10% of the patients will die due to disease complications or the side-effects of the treatment. Targeted therapy may enhance anti-leukemic efficacy and minimize treatment-related morbidity and mortality, but requires detailed knowledge of the genetic abnormalities and aberrant pathways involved in leukemogenesis. These efforts towards future personalized therapy in a rare disease, such as pediatric AML, require intensive international collaboration in order to enhance the survival rates of pediatric AML, while aiming to reduce long-term toxicity.
Keywords: clinical management; cytogenetics; molecular aberrations; pediatric AML.
Figures
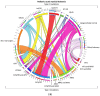

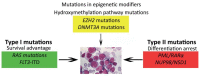
References
-
- Howlader N.N.A., Krapcho M., Garshell J., Miller D., Altekruse S.F., Kosary C.L., Yu M., Ruhl J., Tatalovich Z., Mariotto A., et al. SEER Cancer Statistics Review, 1975–2011. National Cancer Institute; Bethesda, MD, USA: 2012.
-
- Hahn C.N., Chong C.E., Carmichael C.L., Wilkins E.J., Brautigan P.J., Li X.C., Babic M., Lin M., Carmagnac A., Lee Y.K., et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat. Genet. 2011;43:1012–1017. doi: 10.1038/ng.913. - DOI - PMC - PubMed
-
- Link D.C., Schuettpelz L.G., Shen D., Wang J., Walter M.J., Kulkarni S., Payton J.E., Ivanovich J., Goodfellow P.J., Le Beau M., et al. Identification of a novel TP53 cancer susceptibility mutation through whole-genome sequencing of a patient with therapy-related AML. JAMA. 2011;305:1568–1576. doi: 10.1001/jama.2011.473. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

