π-Radical to σ-Radical Tautomerization in One-Electron-Oxidized 1-Methylcytosine and Its Analogs
- PMID: 26237072
- PMCID: PMC4562692
- DOI: 10.1021/acs.jpcb.5b05162
π-Radical to σ-Radical Tautomerization in One-Electron-Oxidized 1-Methylcytosine and Its Analogs
Abstract
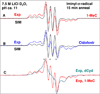
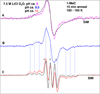
In this work, iminyl σ-radical formation in several one-electron-oxidized cytosine analogs, including 1-MeC, cidofovir, 2'-deoxycytidine (dCyd), and 2'-deoxycytidine 5'-monophosphate (5'-dCMP), were investigated in homogeneous, aqueous (D2O or H2O) glassy solutions at low temperatures by employing electron spin resonance (ESR) spectroscopy. Upon employing density functional theory (DFT) (DFT/B3LYP/6-31G* method), the calculated hyperfine coupling constant (HFCC) values of iminyl σ-radical agree quite well with the experimentally observed ones, thus confirming its assignment. ESR and DFT studies show that the cytosine iminyl σ-radical is a tautomer of the deprotonated cytosine π-cation radical [cytosine π-aminyl radical, C(N4-H)(•)]. Employing 1-MeC samples at various pHs ranging from ca. 8 to 11, ESR studies show that the tautomeric equilibrium between C(N4-H)(•) and the iminyl σ-radical at low temperature is too slow to be established without added base. ESR and DFT studies agree that, in the iminyl σ-radical, the unpaired spin is localized on the exocyclic nitrogen (N4) in an in-plane pure p-orbital. This gives rise to an anisotropic nitrogen hyperfine coupling (Azz = 40 G) from N4 and a near isotropic β-nitrogen coupling of 9.7 G from the cytosine ring nitrogen at N3. Iminyl σ-radical should exist in its N3-protonated form, as the N3-protonated iminyl σ-radical is stabilized in solution by over 30 kcal/mol (ΔG = -32 kcal/mol) over its conjugate base, the N3-deprotonated form. This is the first observation of an isotropic β-hyperfine ring nitrogen coupling in an N-centered DNA radical. Our theoretical calculations predict that the cytosine iminyl σ-radical can be formed in double-stranded DNA by a radiation-induced ionization-deprotonation process that is only 10 kcal/mol above the lowest energy path.
Figures


References
-
- Becker D, Adhikary A, Sevilla MD. The Role of Charge and Spin Migration in DNA Radiation Damage. In: Chakraborty T, editor. Charge Migration in DNA: Physics, Chemistry and Biology Perspectives. Berlin, Heidelberg, New York: Springer-Verlag; 2007. pp. 139–175.
-
- Close DM. From the Primary Radiation Induced Radicals in DNA Constituents to Strand Breaks: Low Temperature EPR/ENDOR studies. In: Shukla MK, Leszczynski J, editors. Radiation-induced Molecular Phenomena in Nucleic Acids: A Comprehensive Theoretical and Experimental Analysis. Berlin, Heidelberg, New York: Springer-Verlag; 2008. pp. 493–529.
-
- Bernhard WA. Radical Reaction Pathways Initiated by Direct Energy Deposition in DNA by Ionizing Radiation. In: Greenberg MM, editor. Radical and Radical Ion Reactivity in Nucleic Acid Chemistry. New Jersey: John Wiley & Sons, Inc.; 2009. pp. 41–68.
-
- Sagstuen E, Hole EO. Radiation Produced Radicals. In: Brustolon, Giamello, editors. Electron Paramagnetic Resonance. New Jersey: John Wiley & Sons, Inc.; 2009. pp. 325–381. (2009)
-
- Becker D, Adhikary A, Sevilla MD. Mechanism of Radiation Induced DNA Damage: Direct Effects. In: Rao BSM, Wishart J, editors. Recent Trends in Radiation Chemistry. Singapore, New Jersey, London: World Scientific Publishing Co.; 2010. pp. 509–542.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

