Mesenchymal Stromal Cells: Updates and Therapeutic Outlook in Rheumatic Diseases
- PMID: 26237144
- PMCID: PMC4470144
- DOI: 10.3390/jcm2040201
Mesenchymal Stromal Cells: Updates and Therapeutic Outlook in Rheumatic Diseases
Abstract
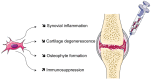
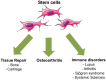
Multipotent mesenchymal stromal cells or mesenchymal stem cells (MSCs) are adult stem cells exhibiting functional properties that have opened the way for cell-based clinical therapies. MSCs have been reported to exhibit immunosuppressive as well as healing properties, improving angiogenesis and preventing apoptosis or fibrosis through the secretion of paracrine mediators. This review summarizes recent progress on the clinical application of stem cells therapy in some inflammatory and degenerative rheumatic diseases. To date, most of the available data have been obtained in preclinical models and clinical efficacy needs to be evaluated through controlled randomized double-blind trials.
Keywords: autoimmune diseases; immune tolerance; mesenchymal stem cells; osteoarthritis; rheumatic diseases; rheumatoid arthritis.
Figures
Similar articles
-
Mesenchymal stromal cells and rheumatic diseases: new tools from pathogenesis to regenerative therapies.Cytotherapy. 2015 Jul;17(7):832-49. doi: 10.1016/j.jcyt.2014.12.006. Epub 2015 Feb 10. Cytotherapy. 2015. PMID: 25680301 Review.
-
Multipotent mesenchymal stromal cells and rheumatoid arthritis: risk or benefit?Rheumatology (Oxford). 2009 Oct;48(10):1185-9. doi: 10.1093/rheumatology/kep162. Epub 2009 Jun 26. Rheumatology (Oxford). 2009. PMID: 19561159 Review.
-
Mesenchymal stem cells and mesenchymal stem cell-derived extracellular vesicles: Potential roles in rheumatic diseases.World J Stem Cells. 2020 Jul 26;12(7):688-705. doi: 10.4252/wjsc.v12.i7.688. World J Stem Cells. 2020. PMID: 32843922 Free PMC article.
-
Inflammatory stimuli differentially modulate the transcription of paracrine signaling molecules of equine bone marrow multipotent mesenchymal stromal cells.Osteoarthritis Cartilage. 2013 Aug;21(8):1116-24. doi: 10.1016/j.joca.2013.05.004. Epub 2013 May 14. Osteoarthritis Cartilage. 2013. PMID: 23685224
-
Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes.Biochimie. 2013 Dec;95(12):2229-34. doi: 10.1016/j.biochi.2013.04.017. Epub 2013 May 16. Biochimie. 2013. PMID: 23685070 Review.
Cited by
-
Meta-analysis of preclinical studies of mesenchymal stromal cells to treat rheumatoid arthritis.EBioMedicine. 2019 Sep;47:563-577. doi: 10.1016/j.ebiom.2019.08.073. Epub 2019 Sep 6. EBioMedicine. 2019. PMID: 31501076 Free PMC article.
References
-
- Pers Y.M., Jorgensen C. Cell and Gene Therapy. In: Bijlsma J.W.J., editor. EULAR Text Book on Rheumatic Diseases. Volume 1. BMJ Publishing Group Ltd.; London, UK: 2012. pp. 1234–1265.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

