Stem Cells on Biomaterials for Synthetic Grafts to Promote Vascular Healing
- PMID: 26237251
- PMCID: PMC4449663
- DOI: 10.3390/jcm3010039
Stem Cells on Biomaterials for Synthetic Grafts to Promote Vascular Healing
Abstract
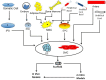
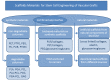
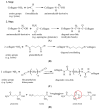
This review is divided into two interconnected parts, namely a biological and a chemical one. The focus of the first part is on the biological background for constructing tissue-engineered vascular grafts to promote vascular healing. Various cell types, such as embryonic, mesenchymal and induced pluripotent stem cells, progenitor cells and endothelial- and smooth muscle cells will be discussed with respect to their specific markers. The in vitro and in vivo models and their potential to treat vascular diseases are also introduced. The chemical part focuses on strategies using either artificial or natural polymers for scaffold fabrication, including decellularized cardiovascular tissue. An overview will be given on scaffold fabrication including conventional methods and nanotechnologies. Special attention is given to 3D network formation via different chemical and physical cross-linking methods. In particular, electron beam treatment is introduced as a method to combine 3D network formation and surface modification. The review includes recently published scientific data and patents which have been registered within the last decade.
Keywords: biomaterial; biopolymer; blood vessel; collagen; cross-linking; endothelial cell; scaffold; smooth muscle cell; stem cell; surface modification.
Figures
Similar articles
-
Surface Modification by Nanobiomaterials for Vascular Tissue Engineering Applications.Curr Med Chem. 2020;27(10):1634-1646. doi: 10.2174/0929867325666180914104633. Curr Med Chem. 2020. PMID: 30215329 Review.
-
Hydrostatic pressure under hypoxia facilitates fabrication of tissue-engineered vascular grafts derived from human vascular smooth muscle cells in vitro.Acta Biomater. 2023 Nov;171:209-222. doi: 10.1016/j.actbio.2023.09.041. Epub 2023 Oct 2. Acta Biomater. 2023. PMID: 37793599
-
Application of 3D-printed tissue-engineered skin substitute using innovative biomaterial loaded with human adipose-derived stem cells in wound healing.Int J Bioprint. 2023 Jan 31;9(2):674. doi: 10.18063/ijb.v9i2.674. eCollection 2023. Int J Bioprint. 2023. PMID: 37065662 Free PMC article.
-
Autologous endothelialized small-caliber vascular grafts engineered from blood-derived induced pluripotent stem cells.Acta Biomater. 2019 Oct 1;97:333-343. doi: 10.1016/j.actbio.2019.07.032. Epub 2019 Jul 22. Acta Biomater. 2019. PMID: 31344511
-
The Advancement of Biomaterials in Regulating Stem Cell Fate.Stem Cell Rev Rep. 2018 Feb;14(1):43-57. doi: 10.1007/s12015-017-9764-y. Stem Cell Rev Rep. 2018. PMID: 28884292 Review.
Cited by
-
Tooth Formation: Are the Hardest Tissues of Human Body Hard to Regenerate?Int J Mol Sci. 2020 Jun 4;21(11):4031. doi: 10.3390/ijms21114031. Int J Mol Sci. 2020. PMID: 32512908 Free PMC article. Review.
-
Small Molecules Enhance Scaffold-Based Bone Grafts via Purinergic Receptor Signaling in Stem Cells.Int J Mol Sci. 2018 Nov 14;19(11):3601. doi: 10.3390/ijms19113601. Int J Mol Sci. 2018. PMID: 30441872 Free PMC article. Review.
-
The role of purinergic receptors in stem cell differentiation.Comput Struct Biotechnol J. 2014 Nov 7;13:75-84. doi: 10.1016/j.csbj.2014.11.003. eCollection 2015. Comput Struct Biotechnol J. 2014. PMID: 26900431 Free PMC article. Review.
-
Role of Hox genes in stem cell differentiation.World J Stem Cells. 2015 Apr 26;7(3):583-95. doi: 10.4252/wjsc.v7.i3.583. World J Stem Cells. 2015. PMID: 25914765 Free PMC article. Review.
-
Zoledronate loaded polylactic acid/polycaprolactone/hydroxyapatite scaffold accelerates regeneration and led to enhance structural performance and functional ability of the radial bone defect in rat.Iran J Vet Res. 2023;24(2):122-125. doi: 10.22099/IJVR.2023.43807.6421. Iran J Vet Res. 2023. PMID: 37790115 Free PMC article.
References
-
- Limbach C.A., Lange M., Schulze M., Tobiasch E. Recent patents on biomedical applications for the treatment of atherosclerosis. Recent Pat. Regen. Med. 2012;2:75–102.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

