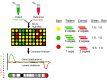
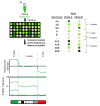
Microarray Technology for the Diagnosis of Fetal Chromosomal Aberrations: Which Platform Should We Use?
- PMID: 26237396
- PMCID: PMC4449692
- DOI: 10.3390/jcm3020663
Microarray Technology for the Diagnosis of Fetal Chromosomal Aberrations: Which Platform Should We Use?
Abstract
The advantage of microarray (array) over conventional karyotype for the diagnosis of fetal pathogenic chromosomal anomalies has prompted the use of microarrays in prenatal diagnostics. In this review we compare the performance of different array platforms (BAC, oligonucleotide CGH, SNP) and designs (targeted, whole genome, whole genome, and targeted, custom) and discuss their advantages and disadvantages in relation to prenatal testing. We also discuss the factors to consider when implementing a microarray testing service for the diagnosis of fetal chromosomal aberrations.
Keywords: BAC; SNP; implementation; prenatal microarray.
Figures
References
-
- Miller D.T., Adam M.P., Aradhya S., Biesecker L.G., Brothman A.R., Carter N.P., Church D.M., Crolla J.A., Eichler E.E., Epstein C.J., et al. Consensus statement: Chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am. J. Hum. Genet. 2010;86:749–764. doi: 10.1016/j.ajhg.2010.04.006. - DOI - PMC - PubMed
-
- Shaffer L.G., Dabell M.P., Fisher A.J., Coppinger J., Bandholz A.M., Ellison J.W., Ravnan J.B., Torchia B.S., Ballif B.C., Rosenfeld J.A. Experience with microarray-based comparative genomic hybridization for prenatal diagnosis in over 5000 pregnancies. Prenat. Diagn. 2012;32:976–985. doi: 10.1002/pd.3945. - DOI - PMC - PubMed
-
- Fiorentino F., Napoletano S., Caiazzo F., Sessa M., Bono S., Spizzichino L., Gordon A., Nuccitelli A., Rizzo G., Baldi M. Chromosomal microarray analysis as a first-line test in pregnancies with a priori low risk for the detection of submicroscopic chromosomal abnormalities. Eur. J. Hum. Genet. 2013;21:725–730. doi: 10.1038/ejhg.2012.253. - DOI - PMC - PubMed
-
- Callaway J.L., Shaffer L.G., Chitty L.S., Rosenfeld J.A., Crolla J.A. The clinical utility of microarray technologies applied to prenatal cytogenetics in the presence of a normal conventional karyotype: A review of the literature. Prenat. Diagn. 2013;33:1119–1123. doi: 10.1002/pd.4209. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources