Serious Adverse Events in the Canadian Registry of Children Receiving Palivizumab (CARESS) for Respiratory Syncytial Virus Prevention
- PMID: 26237402
- PMCID: PMC4523213
- DOI: 10.1371/journal.pone.0134711
Serious Adverse Events in the Canadian Registry of Children Receiving Palivizumab (CARESS) for Respiratory Syncytial Virus Prevention
Abstract
Objectives: To evaluate the safety and tolerability of palivizumab for RSV prophylaxis in high-risk children in everyday practice.
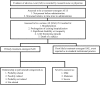
Methods: High-risk children prophylaxed against RSV infection were recruited into a prospective, observational, Canadian RSV Evaluation Study of Palivizumab (CARESS) registry with active, serious adverse event (SAE) monitoring from 2008 to 2013. SAE reports were systematically collected and assessed for severity and relationship to palivizumab. Data were analyzed by Chi-square or Fisher Exact Tests to examine group differences in proportions.
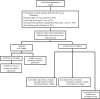
Results: 13025 infants received 57392 injections. Hospitalizations for respiratory-related illness (RIH) were reported in 915 patients, and SAEs other than RIH were reported in 52 patients. Of these, 6 (0.05%) patients had a total of 14 hypersensitivity reactions that were deemed possibly or probably related to palivizumab (incidence: 2.8 per 10,000 patient-months). The SAEs of 42 patients were assessed as not related to palivizumab. SAEs in the remaining 4 patients were not classifiable as their records were incomplete. There were no significant demographic predictors of SAE occurrence.
Conclusions: Under active surveillance, a small proportion of infants in the CARESS registry experienced SAEs that had a potential relationship with palivizumab and these appeared to be unpredictable in terms of onset. Palivizumab appears to be a safe and well-tolerated antibody for RSV prophylaxis in high-risk children in routine practice.
Conflict of interest statement
Figures
Similar articles
-
Respiratory Syncytial Virus Prophylaxis in Infants With Congenital Diaphragmatic Hernia in the Canadian Respiratory Syncytial Virus Evaluation Study of Palivizumab, 2005-2017.Clin Infect Dis. 2019 Aug 30;69(6):980-986. doi: 10.1093/cid/ciy1010. Clin Infect Dis. 2019. PMID: 30517603 Free PMC article.
-
Palivizumab prophylaxis for respiratory syncytial virus in infants with cystic fibrosis: is there a need?Eur J Clin Microbiol Infect Dis. 2018 Jun;37(6):1113-1118. doi: 10.1007/s10096-018-3225-7. Epub 2018 Mar 19. Eur J Clin Microbiol Infect Dis. 2018. PMID: 29557081
-
First versus second year respiratory syncytial virus prophylaxis in chronic lung disease (2005-2015).Eur J Pediatr. 2017 Mar;176(3):413-422. doi: 10.1007/s00431-017-2849-4. Epub 2017 Jan 20. Eur J Pediatr. 2017. PMID: 28105526 Free PMC article.
-
Product review on the monoclonal antibody palivizumab for prevention of respiratory syncytial virus infection.Hum Vaccin Immunother. 2017 Sep 2;13(9):2138-2149. doi: 10.1080/21645515.2017.1337614. Epub 2017 Jun 12. Hum Vaccin Immunother. 2017. PMID: 28605249 Free PMC article. Review.
-
Impact of the 2014 American Academy of Pediatrics recommendation and of the resulting limited financial coverage by the Italian Medicines Agency for palivizumab prophylaxis on the RSV-associated hospitalizations in preterm infants during the 2016-2017 epidemic season: a systematic review of seven Italian reports.Ital J Pediatr. 2019 Nov 9;45(1):139. doi: 10.1186/s13052-019-0736-5. Ital J Pediatr. 2019. PMID: 31706338 Free PMC article.
Cited by
-
Past, Present and Future Approaches to the Prevention and Treatment of Respiratory Syncytial Virus Infection in Children.Infect Dis Ther. 2018 Mar;7(1):87-120. doi: 10.1007/s40121-018-0188-z. Epub 2018 Feb 22. Infect Dis Ther. 2018. PMID: 29470837 Free PMC article. Review.
-
Immunological Features of Respiratory Syncytial Virus-Caused Pneumonia-Implications for Vaccine Design.Int J Mol Sci. 2017 Mar 4;18(3):556. doi: 10.3390/ijms18030556. Int J Mol Sci. 2017. PMID: 28273842 Free PMC article. Review.
-
Respiratory syncytial virus hospitalizations in US preterm infants after the 2014 change in immunoprophylaxis guidance by the American Academy of Pediatrics.J Perinatol. 2020 Aug;40(8):1135-1144. doi: 10.1038/s41372-020-0689-y. Epub 2020 Jun 4. J Perinatol. 2020. PMID: 32499597 Free PMC article. Review.
-
Hypersensitivity reactions to biologics (part I): allergy as an important differential diagnosis in complex immune-derived adverse events.Allergo J. 2020;29(4):32-61. doi: 10.1007/s15007-020-2550-1. Epub 2020 Jun 24. Allergo J. 2020. PMID: 32546899 Free PMC article. German.
-
Respiratory syncytial virus hospitalization and incurred morbidities the season after prophylaxis.Paediatr Child Health. 2018 Nov;23(7):441-446. doi: 10.1093/pch/pxy046. Epub 2018 Apr 14. Paediatr Child Health. 2018. PMID: 30374219 Free PMC article.
References
-
- Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986; 140: 543–546. - PubMed
-
- Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr 1981; 98: 708–715. - PubMed
-
- Collins CL, Pollard AJ. Respiratory syncytial virus infections in children and adults. J Infect. 2002; 45: 10–17. - PubMed
-
- Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001; 344: 1917–1928. - PubMed
-
- Regnier SA, Huels J. Association between respiratory syncytial virus hospitalizations in infants and respiratory sequelae: systematic review and meta-analysis. Pediatr Infect Dis J. 2013; 32: 820–826. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

