High-throughput determination of RNA structure by proximity ligation
- PMID: 26237516
- PMCID: PMC4564351
- DOI: 10.1038/nbt.3289
High-throughput determination of RNA structure by proximity ligation
Abstract
We present an unbiased method to globally resolve RNA structures through pairwise contact measurements between interacting regions. RNA proximity ligation (RPL) uses proximity ligation of native RNA followed by deep sequencing to yield chimeric reads with ligation junctions in the vicinity of structurally proximate bases. We apply RPL in both baker's yeast (Saccharomyces cerevisiae) and human cells and generate contact probability maps for ribosomal and other abundant RNAs, including yeast snoRNAs, the RNA subunit of the signal recognition particle and the yeast U2 spliceosomal RNA homolog. RPL measurements correlate with established secondary structures for these RNA molecules, including stem-loop structures and long-range pseudoknots. We anticipate that RPL will complement the current repertoire of computational and experimental approaches in enabling the high-throughput determination of secondary and tertiary RNA structures.
Figures
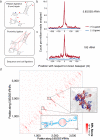
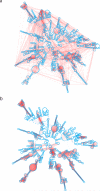


Comment in
-
Biochemistry: cut and paste for RNA structure.Nat Methods. 2015 Oct;12(10):906-7. doi: 10.1038/nmeth.3606. Nat Methods. 2015. PMID: 26688884 No abstract available.
References
-
- Mortimer SA, Kidwell MA, Doudna JA. Insights into RNA structure and function from genome-wide studies. Nat. Rev. Genet. 2014;15:469–479. CrossRef Medline. - PubMed
-
- Cate JH, et al. Crystal Structure of a Group I Ribozyme Domain: Principles of RNA Packing. Science. 1996;273:1678–1685. CrossRef Medline. - PubMed
-
- Wang Y-H, Murphy FL, Cech TR, Griffith JD. Visualization of a Tertiary Structural Domain of the Tetrahymena Group I Intron by Electron Microscopy. J. Mol. Biol. 1994;236:64–71. CrossRef Medline. - PubMed
-
- Latham MP, Brown DJ, McCallum SA, Pardi A. NMR Methods for Studying the Structure and Dynamics of RNA. ChemBioChem. 2005;6:1492–1505. CrossRef Medline. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

