A Photostable Silicon Rhodamine Platform for Optical Voltage Sensing
- PMID: 26237573
- PMCID: PMC4666802
- DOI: 10.1021/jacs.5b06644
A Photostable Silicon Rhodamine Platform for Optical Voltage Sensing
Abstract
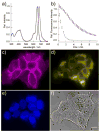
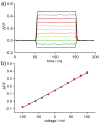
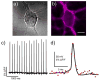
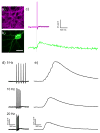
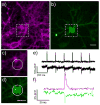
This paper describes the design and synthesis of a photostable, far-red to near-infrared (NIR) platform for optical voltage sensing. We developed a new, sulfonated silicon rhodamine fluorophore and integrated it with a phenylenevinylene molecular wire to create a Berkeley Red Sensor of Transmembrane potential, or BeRST 1 ("burst"). BeRST 1 is the first member of a class of far-red to NIR voltage sensitive dyes that make use of a photoinduced electron transfer (PeT) trigger for optical interrogation of membrane voltage. We show that BeRST 1 displays bright, membrane-localized fluorescence in living cells, high photostability, and excellent voltage sensitivity in neurons. Depolarization of the plasma membrane results in rapid fluorescence increases (24% ΔF/F per 100 mV). BeRST 1 can be used in conjunction with fluorescent stains for organelles, Ca(2+) indicators, and voltage-sensitive fluorescent proteins. In addition, the red-shifted spectral profile of BeRST 1, relative to commonly employed optogenetic actuators like ChannelRhodopsin2 (ChR2), which require blue light, enables optical electrophysiology in neurons. The high speed, sensitivity, photostability and long-wavelength fluorescence profiles of BeRST 1 make it a useful platform for the noninvasive, optical dissection of neuronal activity.
Figures

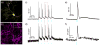
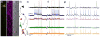

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

