Defects in DNA Repair Genes Predict Response to Neoadjuvant Cisplatin-based Chemotherapy in Muscle-invasive Bladder Cancer
- PMID: 26238431
- PMCID: PMC4764095
- DOI: 10.1016/j.eururo.2015.07.009
Defects in DNA Repair Genes Predict Response to Neoadjuvant Cisplatin-based Chemotherapy in Muscle-invasive Bladder Cancer
Abstract
Background: Cisplatin-based neoadjuvant chemotherapy (NAC) before cystectomy is the standard of care for muscle-invasive bladder cancer (MIBC), with 25-50% of patients expected to achieve a pathologic response. Validated biomarkers predictive of response are currently lacking.
Objective: To discover and validate biomarkers predictive of response to NAC for MIBC.
Design, setting, and participants: Pretreatment MIBC samples prospectively collected from patients treated in two separate clinical trials of cisplatin-based NAC provided the discovery and validation sets. DNA from pretreatment tumor tissue was sequenced for all coding exons of 287 cancer-related genes and was analyzed for base substitutions, indels, copy number alterations, and selected rearrangements in a Clinical Laboratory Improvements Amendments-certified laboratory.
Outcome measurements and statistical analysis: The mean number of variants and variant status for each gene were correlated with response. Variant data from the discovery cohort were used to create a classification tree to discriminate responders from nonresponders. The resulting decision rule was then tested in the independent validation set.
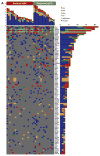
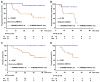
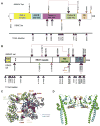
Results and limitations: Patients with a pathologic complete response had more alterations than those with residual tumor in both the discovery (p=0.024) and validation (p=0.018) sets. In the discovery set, alteration in one or more of the three DNA repair genes ATM, RB1, and FANCC predicted pathologic response (p<0.001; 87% sensitivity, 100% specificity) and better overall survival (p=0.007). This test remained predictive for pathologic response in the validation set (p=0.033), with a trend towards better overall survival (p=0.055). These results require further validation in additional sample sets.
Conclusions: Genomic alterations in the DNA repair-associated genes ATM, RB1, and FANCC predict response and clinical benefit after cisplatin-based chemotherapy for MIBC. The results suggest that defective DNA repair renders tumors sensitive to cisplatin.
Patient summary: Chemotherapy given before bladder removal (cystectomy) improves the chance of cure for some but not all patients with muscle-invasive bladder cancer. We found a set of genetic mutations that when present in tumor tissue predict benefit from neoadjuvant chemotherapy, suggesting that testing before chemotherapy may help in selecting patients for whom this approach is recommended.
Keywords: ATM; Biomarkers; Bladder cancer; Cisplatin resistance; Cisplatin sensitivity; DNA repair; FANCC; Neoadjuvant chemotherapy; RB1; Urothelial carcinoma.
Copyright © 2015 European Association of Urology. Published by Elsevier B.V. All rights reserved.
Figures

Comment in
-
Three Genes to Predict Response to Chemotherapy for Bladder Cancer: Individualised Cancer Care at the Doorstep.Eur Urol. 2015 Dec;68(6):968-9. doi: 10.1016/j.eururo.2015.07.050. Epub 2015 Aug 13. Eur Urol. 2015. PMID: 26278806 No abstract available.
-
Bladder cancer: A step closer to individualized treatment for bladder cancer.Nat Rev Urol. 2016 Mar;13(3):127-8. doi: 10.1038/nrurol.2015.265. Epub 2015 Nov 24. Nat Rev Urol. 2016. PMID: 26597618 No abstract available.
Similar articles
-
Genomic Differences Between "Primary" and "Secondary" Muscle-invasive Bladder Cancer as a Basis for Disparate Outcomes to Cisplatin-based Neoadjuvant Chemotherapy.Eur Urol. 2019 Feb;75(2):231-239. doi: 10.1016/j.eururo.2018.09.002. Epub 2018 Oct 2. Eur Urol. 2019. PMID: 30290956 Free PMC article.
-
Defects in DNA Repair Genes Confer Improved Long-term Survival after Cisplatin-based Neoadjuvant Chemotherapy for Muscle-invasive Bladder Cancer.Eur Urol Oncol. 2020 Aug;3(4):544-547. doi: 10.1016/j.euo.2020.02.003. Epub 2020 Mar 10. Eur Urol Oncol. 2020. PMID: 32165095 Free PMC article.
-
Assessment of Predictive Genomic Biomarkers for Response to Cisplatin-based Neoadjuvant Chemotherapy in Bladder Cancer.Eur Urol. 2023 Apr;83(4):313-317. doi: 10.1016/j.eururo.2022.07.023. Epub 2022 Aug 11. Eur Urol. 2023. PMID: 35965206
-
Forty years of cisplatin-based chemotherapy in muscle-invasive bladder cancer: are we understanding how, who and when?World J Urol. 2019 Sep;37(9):1759-1765. doi: 10.1007/s00345-018-2544-8. Epub 2018 Nov 3. World J Urol. 2019. PMID: 30392011 Review.
-
Using the neoadjuvant chemotherapy paradigm to develop precision therapy for muscle-invasive bladder cancer.Urol Oncol. 2016 Oct;34(10):469-76. doi: 10.1016/j.urolonc.2016.05.012. Epub 2016 Jun 15. Urol Oncol. 2016. PMID: 27317490 Review.
Cited by
-
Significance and Mechanisms Analyses of RB1 Mutation in Bladder Cancer Disease Progression and Drug Selection by Bioinformatics Analysis.Bladder Cancer. 2021 May 25;7(2):133-142. doi: 10.3233/BLC-200368. eCollection 2021. Bladder Cancer. 2021. PMID: 38994537 Free PMC article.
-
Genomic characterization of response to chemoradiation in urothelial bladder cancer.Cancer. 2016 Dec 1;122(23):3715-3723. doi: 10.1002/cncr.30219. Epub 2016 Aug 1. Cancer. 2016. PMID: 27479538 Free PMC article.
-
A novel signature to predict the neoadjuvant chemotherapy response of bladder carcinoma: Results from a territory multicenter real-world study.Front Genet. 2022 Nov 2;13:1047481. doi: 10.3389/fgene.2022.1047481. eCollection 2022. Front Genet. 2022. PMID: 36406127 Free PMC article.
-
Elevated Derived Neutrophil-to-Lymphocyte Ratio Corresponds With Poor Outcome in Patients Undergoing Pre-Operative Chemotherapy in Muscle-Invasive Bladder Cancer.Bladder Cancer. 2016 Jul 27;2(3):351-360. doi: 10.3233/BLC-160055. Bladder Cancer. 2016. PMID: 27500202 Free PMC article.
-
Neoadjuvant Dose-dense Gemcitabine and Cisplatin in Muscle-invasive Bladder Cancer: Results of a Phase 2 Trial.Eur Urol Oncol. 2018 May;1(1):54-60. doi: 10.1016/j.euo.2018.02.007. Epub 2018 Jun 6. Eur Urol Oncol. 2018. PMID: 30420974 Free PMC article.
References
-
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. - PubMed
-
- Vale CL. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48:202–6. - PubMed
-
- Plimack ER, Hoffman-Censits JH, Viterbo R, et al. Accelerated meth-otrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol. 2014;32:1895–901. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

