The Focinator - a new open-source tool for high-throughput foci evaluation of DNA damage
- PMID: 26238507
- PMCID: PMC4554354
- DOI: 10.1186/s13014-015-0453-1
The Focinator - a new open-source tool for high-throughput foci evaluation of DNA damage
Abstract
Background: The quantitative analysis of foci plays an important role in many cell biological methods such as counting of colonies or cells, organelles or vesicles, or the number of protein complexes. In radiation biology and molecular radiation oncology, DNA damage and DNA repair kinetics upon ionizing radiation (IR) are evaluated by counting protein clusters or accumulations of phosphorylated proteins recruited to DNA damage sites. Consistency in counting and interpretation of foci remains challenging. Many current software solutions describe instructions for time-consuming and error-prone manual analysis, provide incomplete algorithms for analysis or are expensive. Therefore, we aimed to develop a tool for costless, automated, quantitative and qualitative analysis of foci.
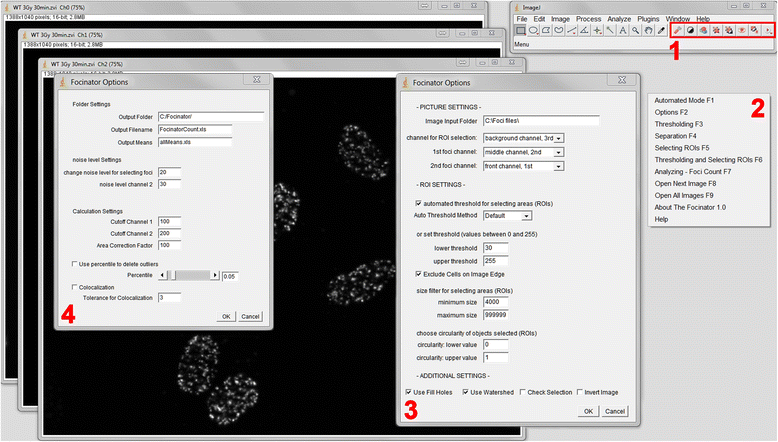
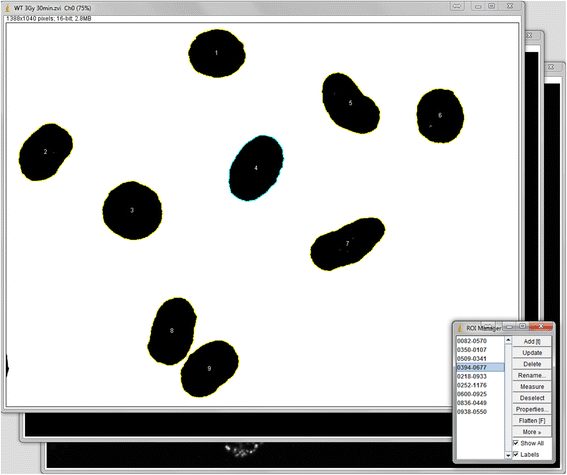
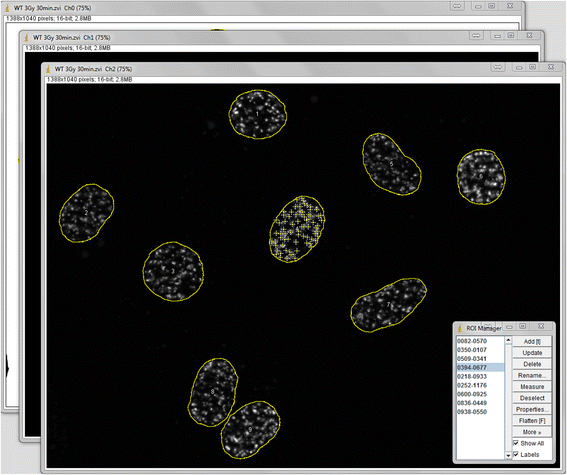
Methods: For this purpose we integrated a user-friendly interface into ImageJ and selected parameters to allow automated selection of regions of interest (ROIs) depending on their size and circularity. We added different export options and a batch analysis. The use of the Focinator was tested by analyzing γ-H2.AX foci in murine prostate adenocarcinoma cells (TRAMP-C1) at different time points after IR with 0.5 to 3 Gray (Gy). Additionally, measurements were performed by users with different backgrounds and experience.
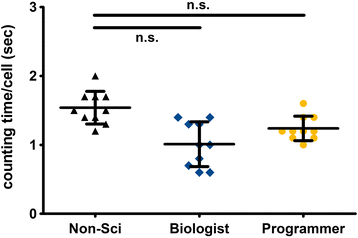
Results: The Focinator turned out to be an easily adjustable tool for automation of foci counting. It significantly reduced the analysis time of radiation-induced DNA-damage foci. Furthermore, different user groups were able to achieve a similar counting velocity. Importantly, there was no difference in nuclei detection between the Focinator and ImageJ alone.
Conclusions: The Focinator is a costless, user-friendly tool for fast high-throughput evaluation of DNA repair foci. The macro allows improved foci evaluation regarding accuracy, reproducibility and analysis speed compared to manual analysis. As innovative option, the macro offers a combination of multichannel evaluation including colocalization analysis and the possibility to run all analyses in a batch mode.
Figures


References
-
- Hašek J, Streiblová E. Fluorescence microscopy methods. In: Evans I, editor. Yeast protocols. Methods in molecular biology™. Totowa, NJ: Humana Press; 1996. pp. 391–405. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

